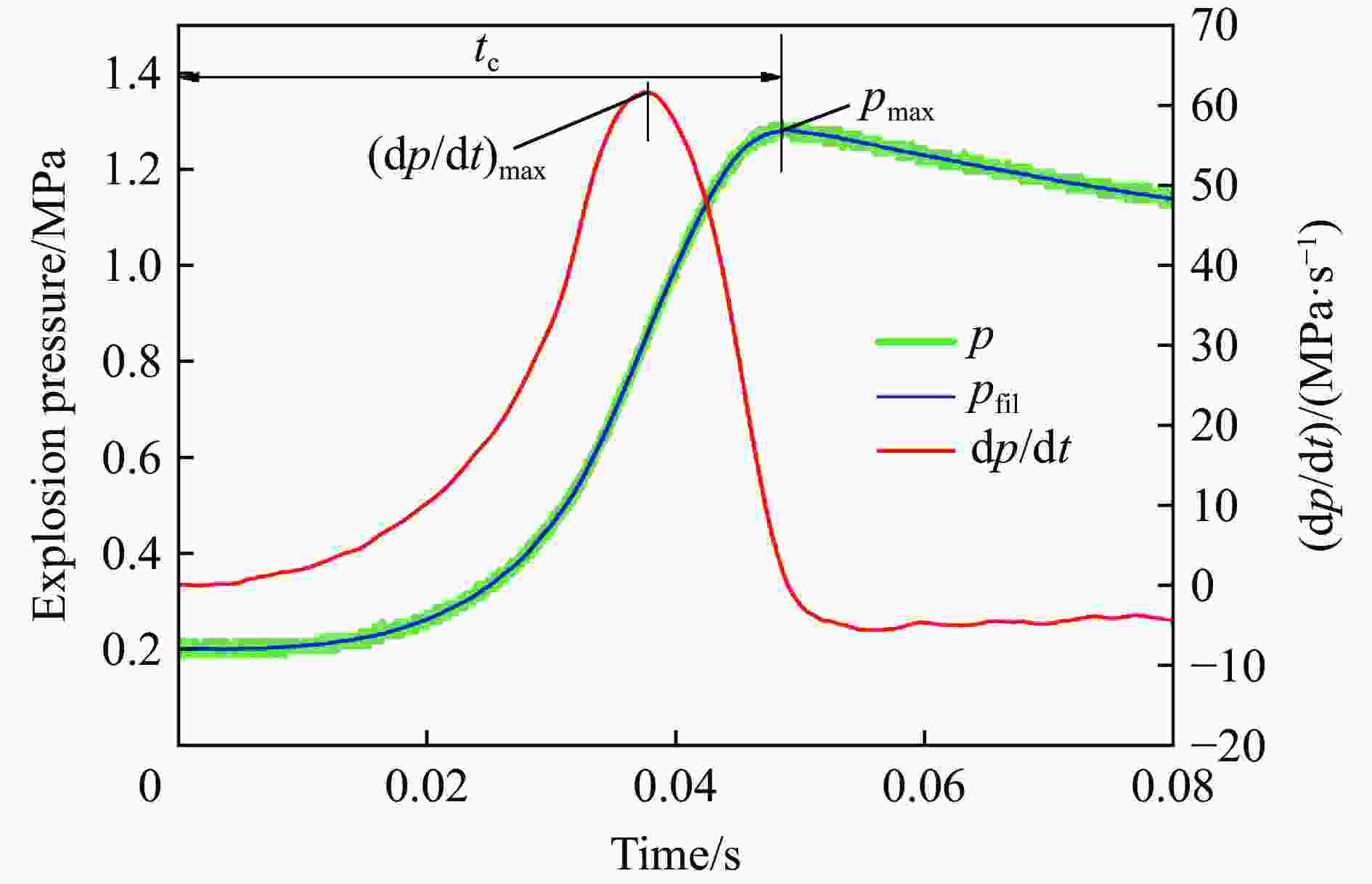
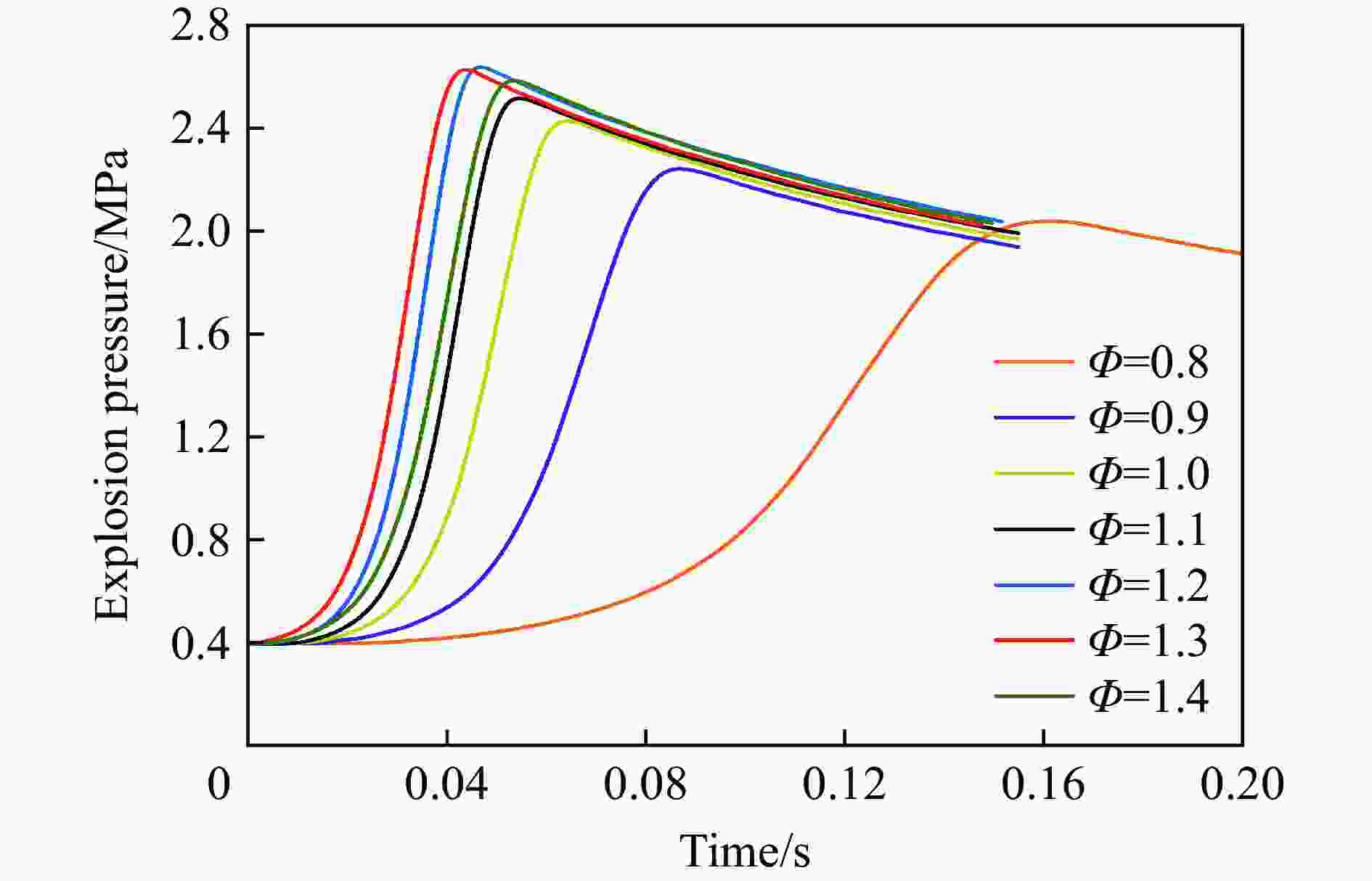
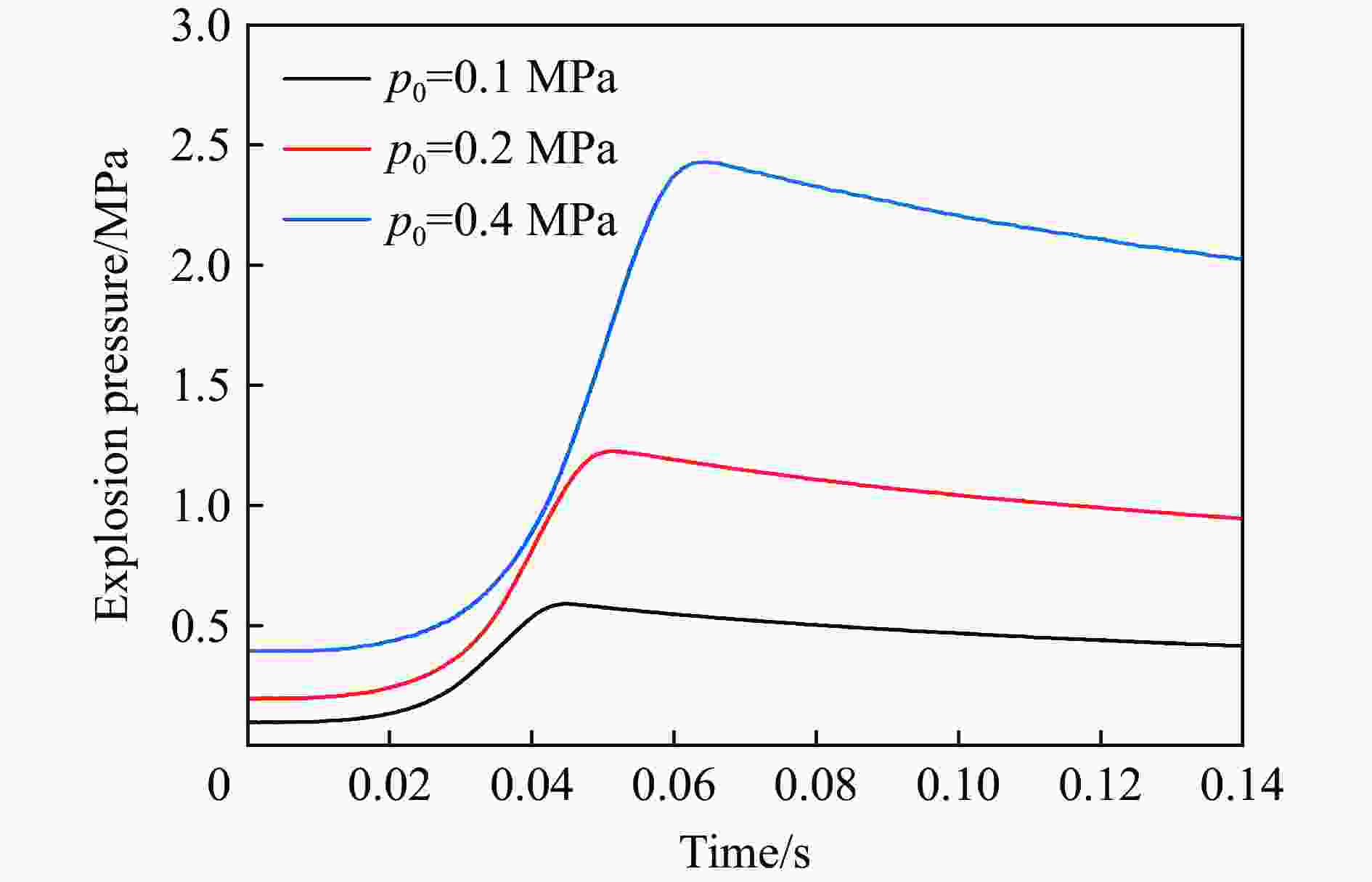
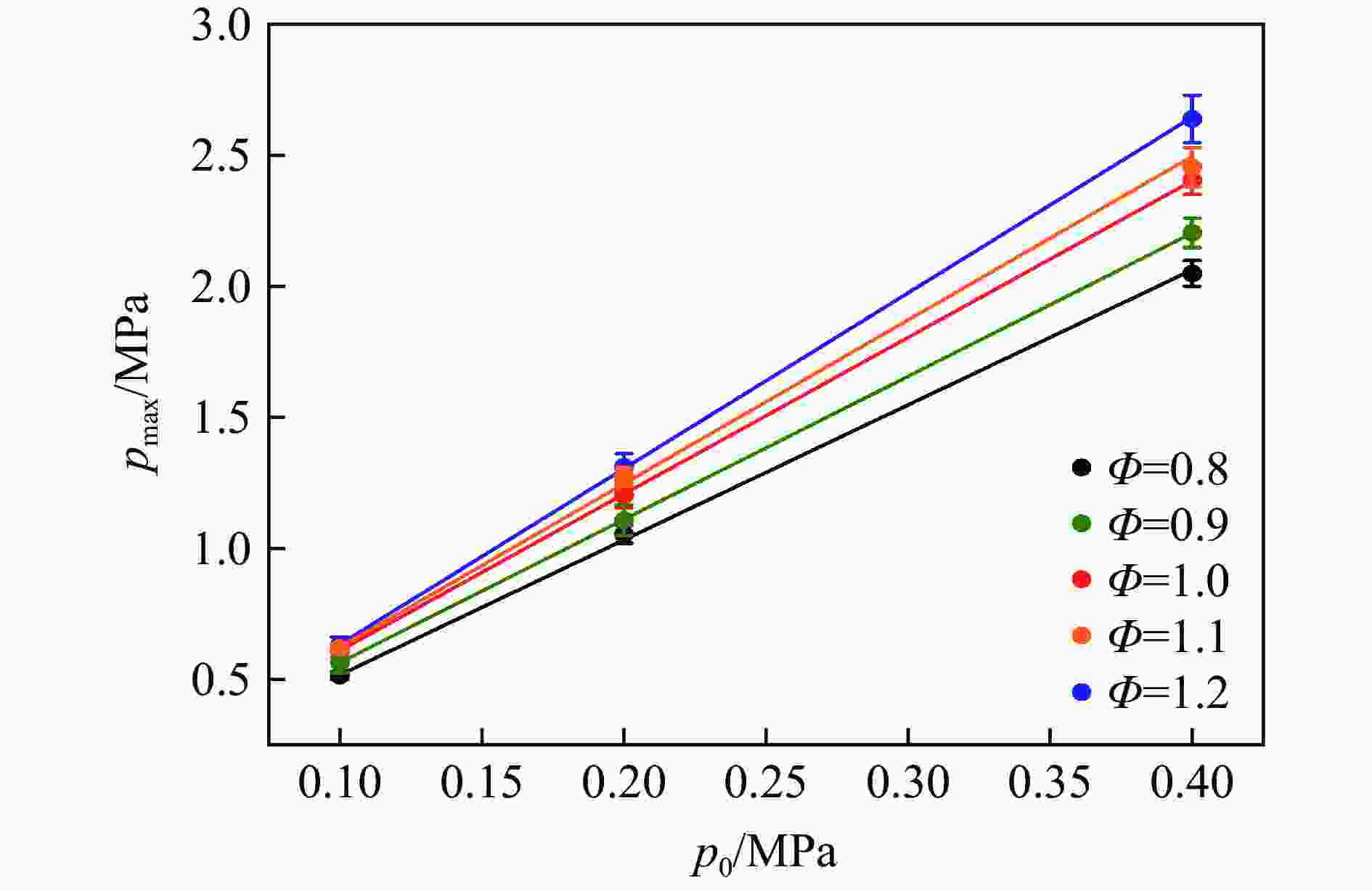
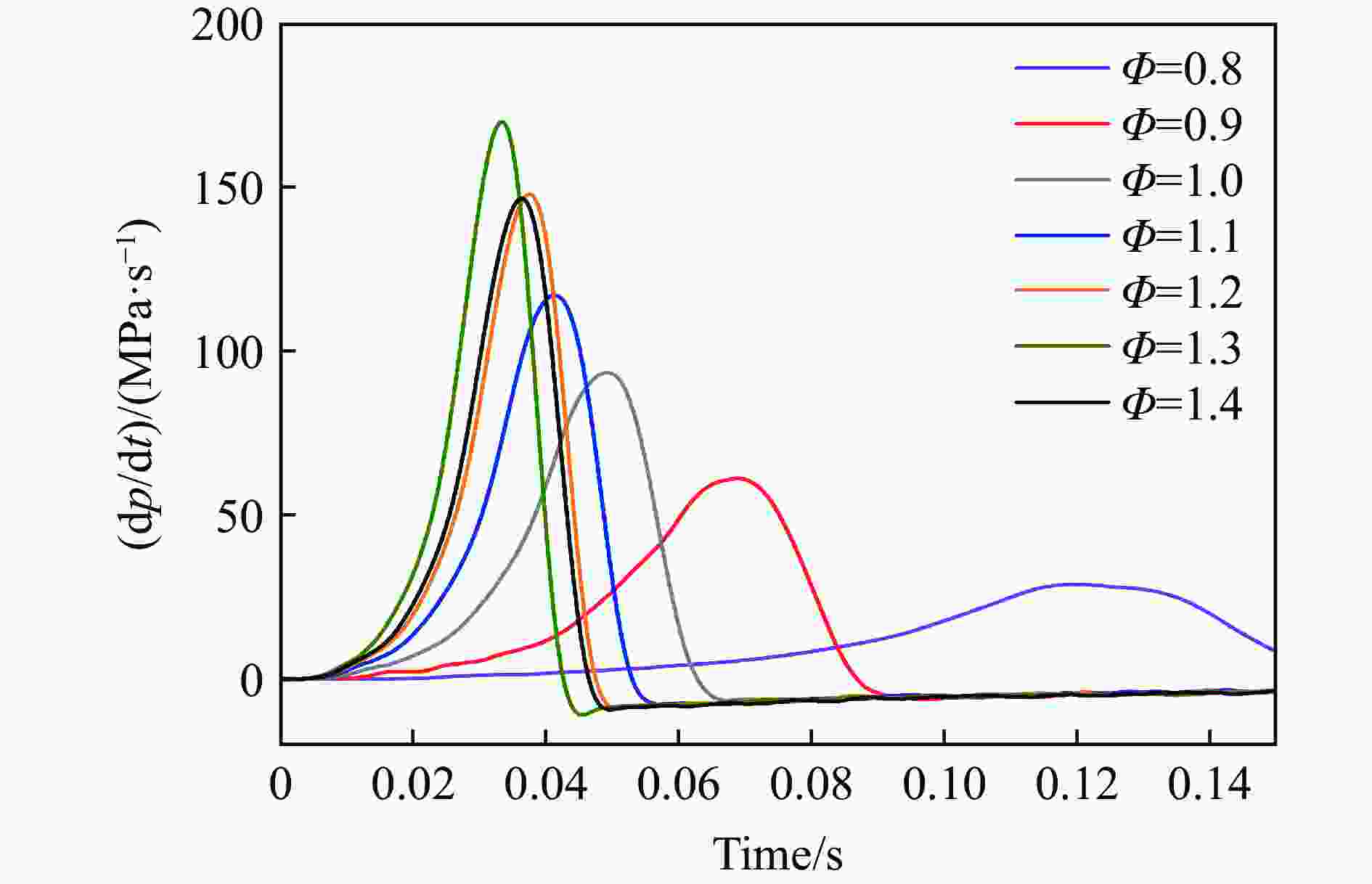
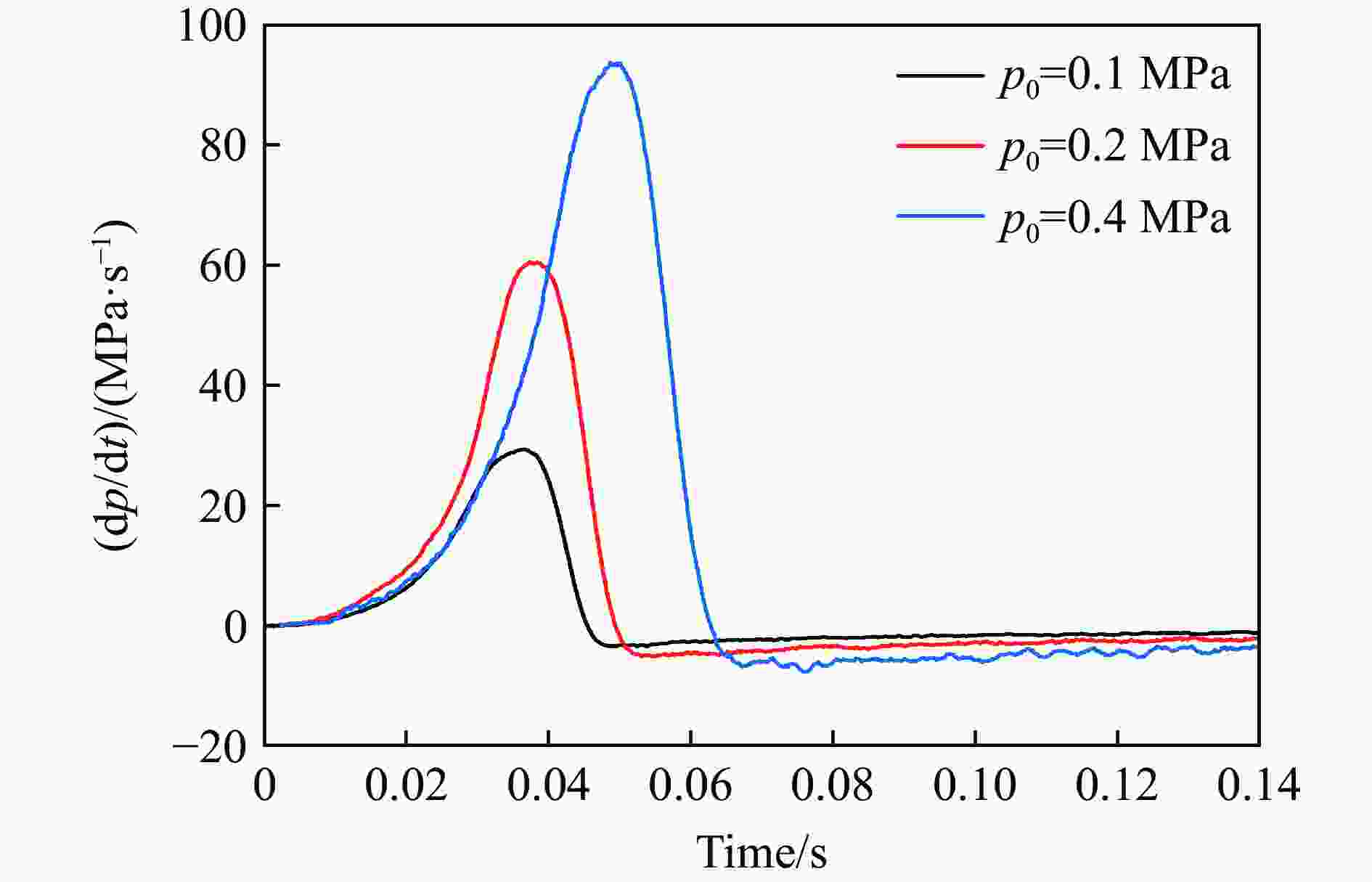
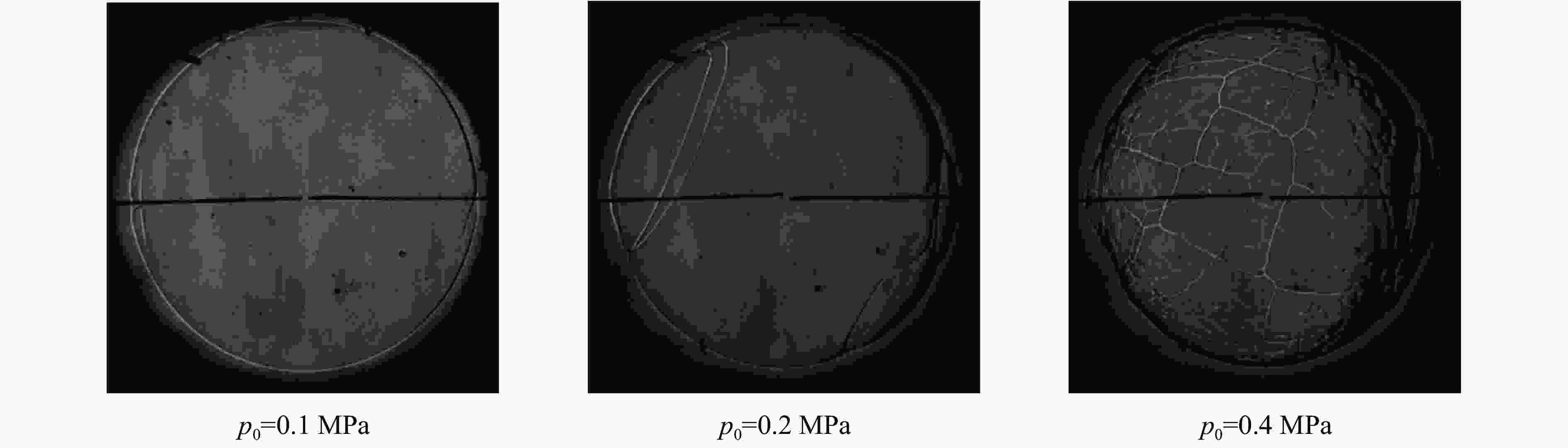
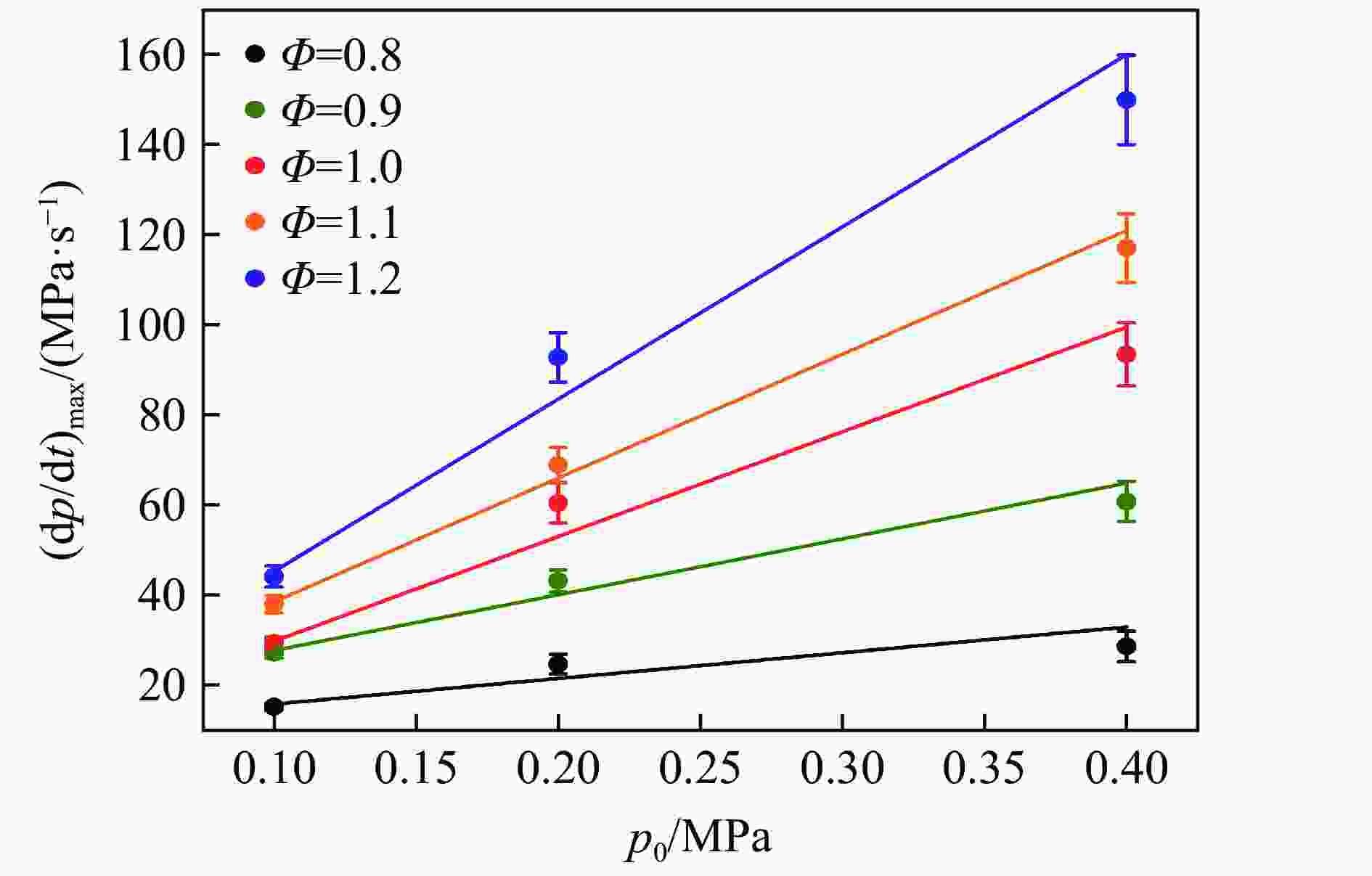
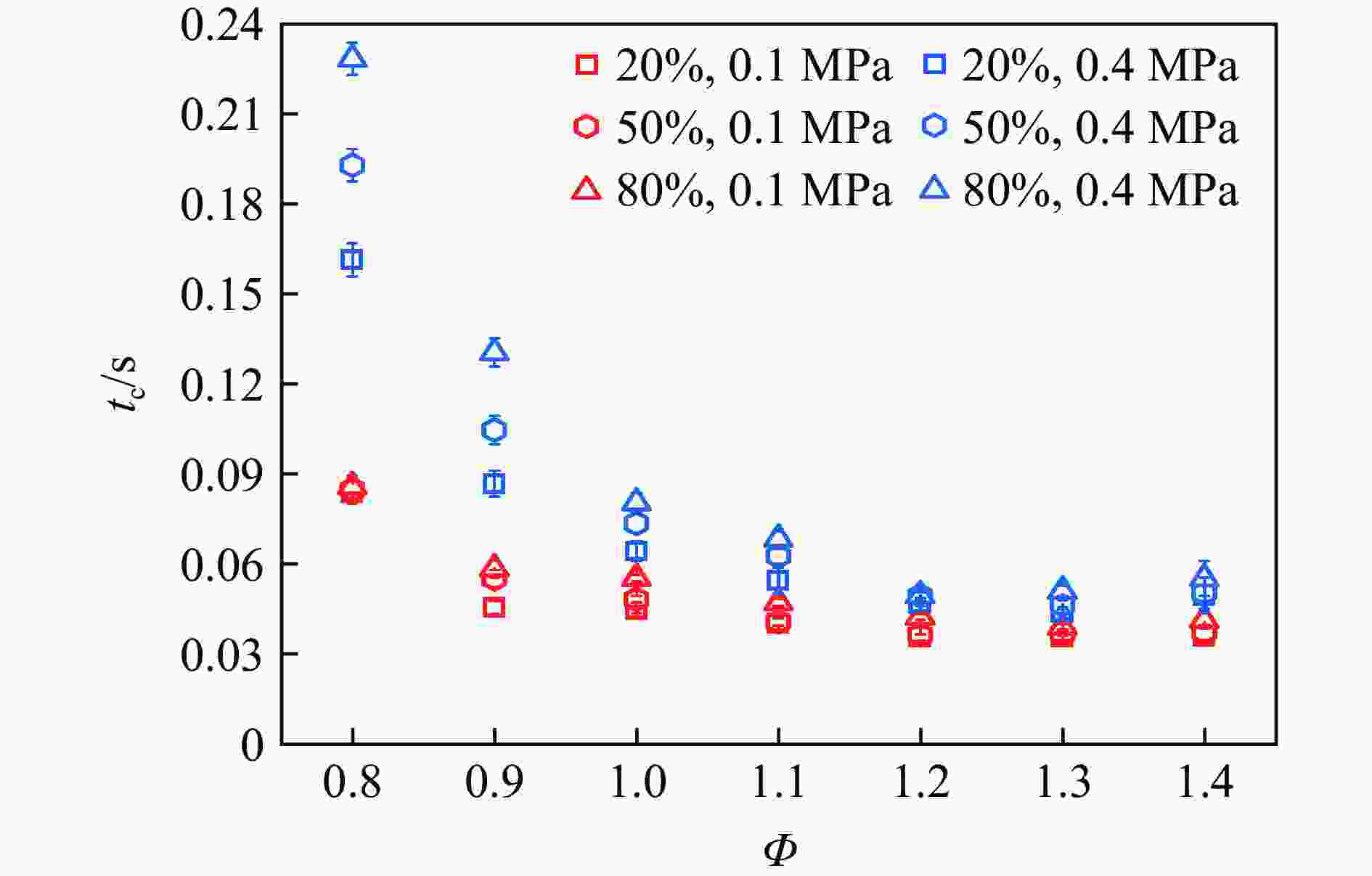
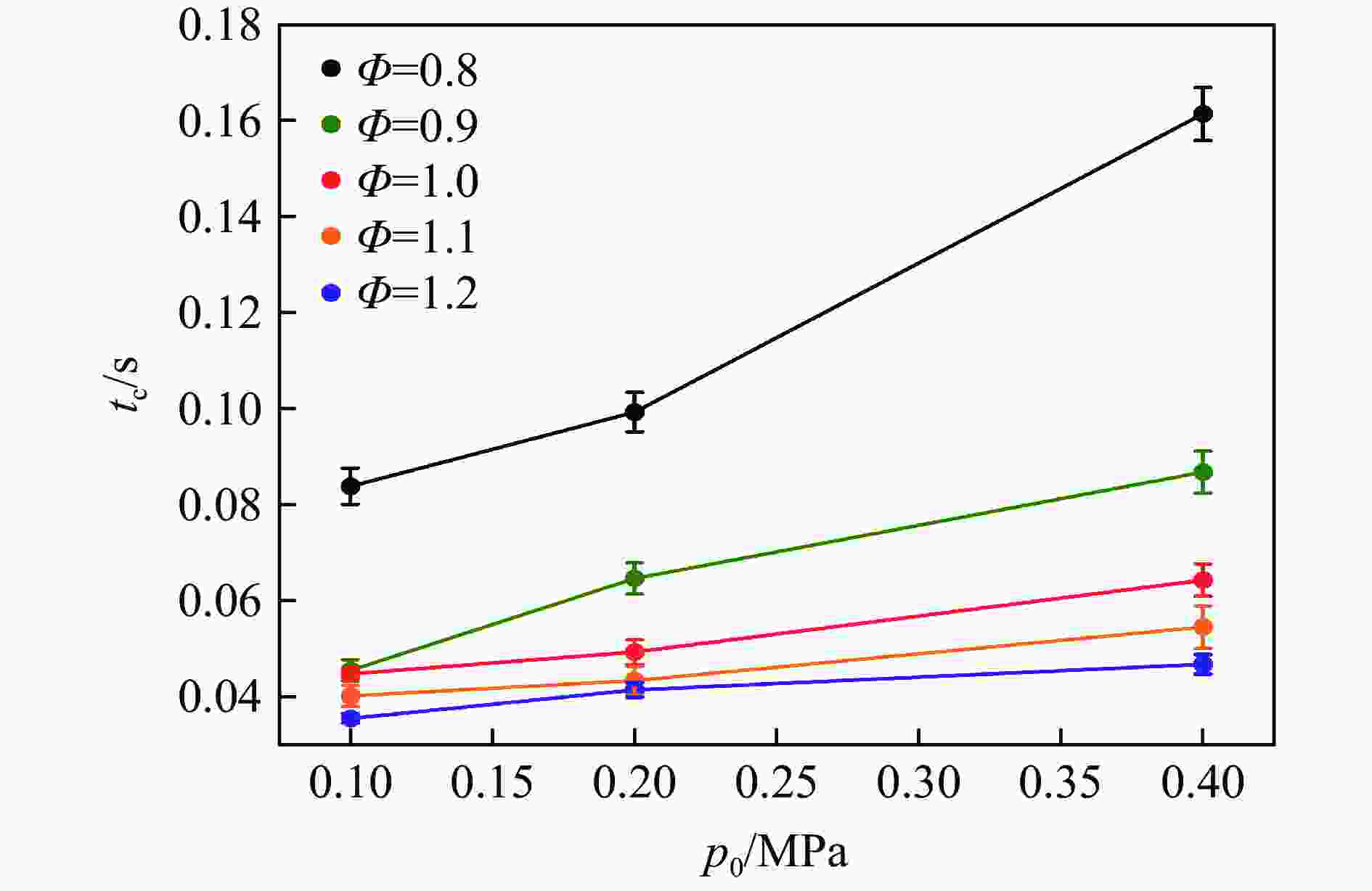
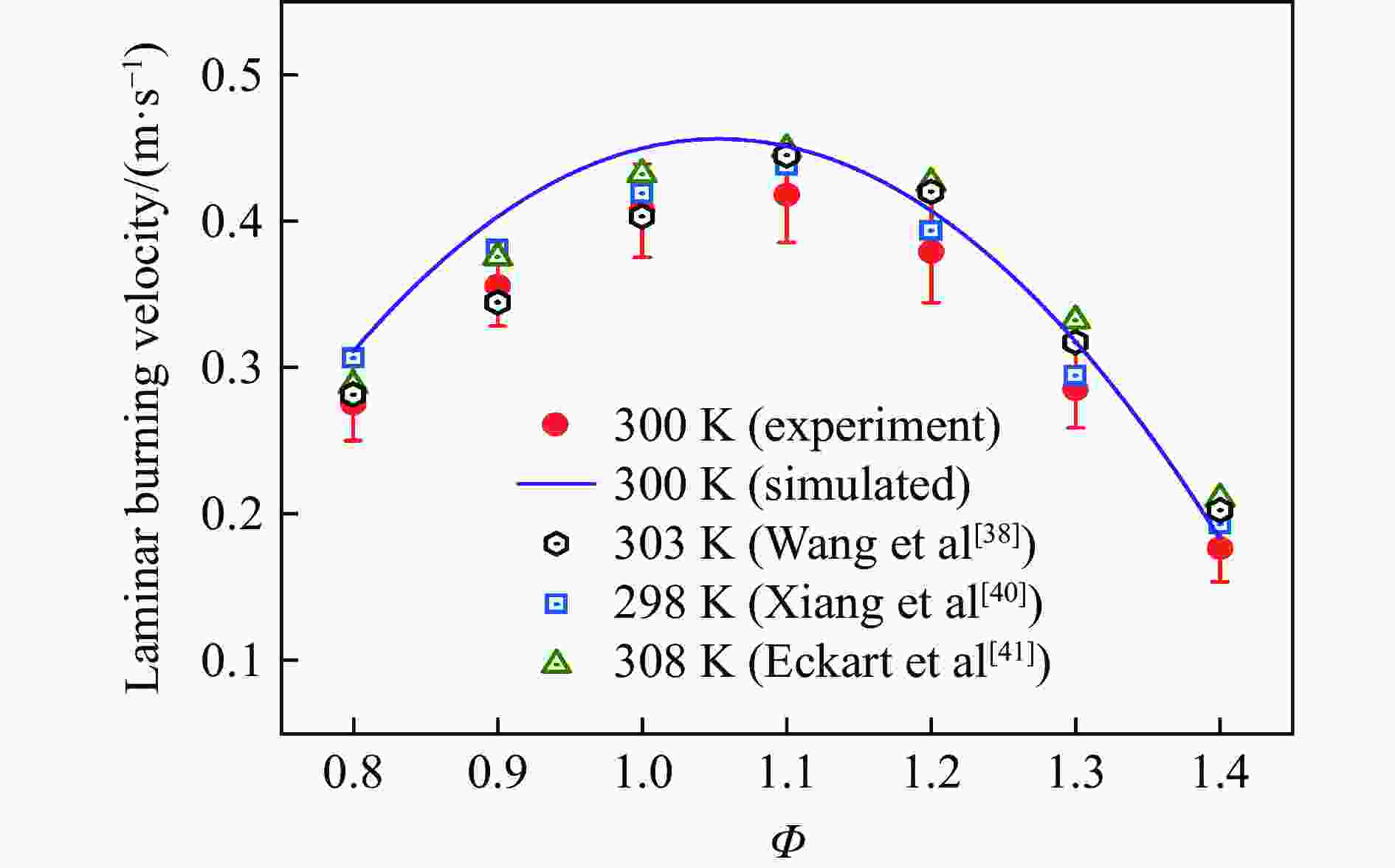
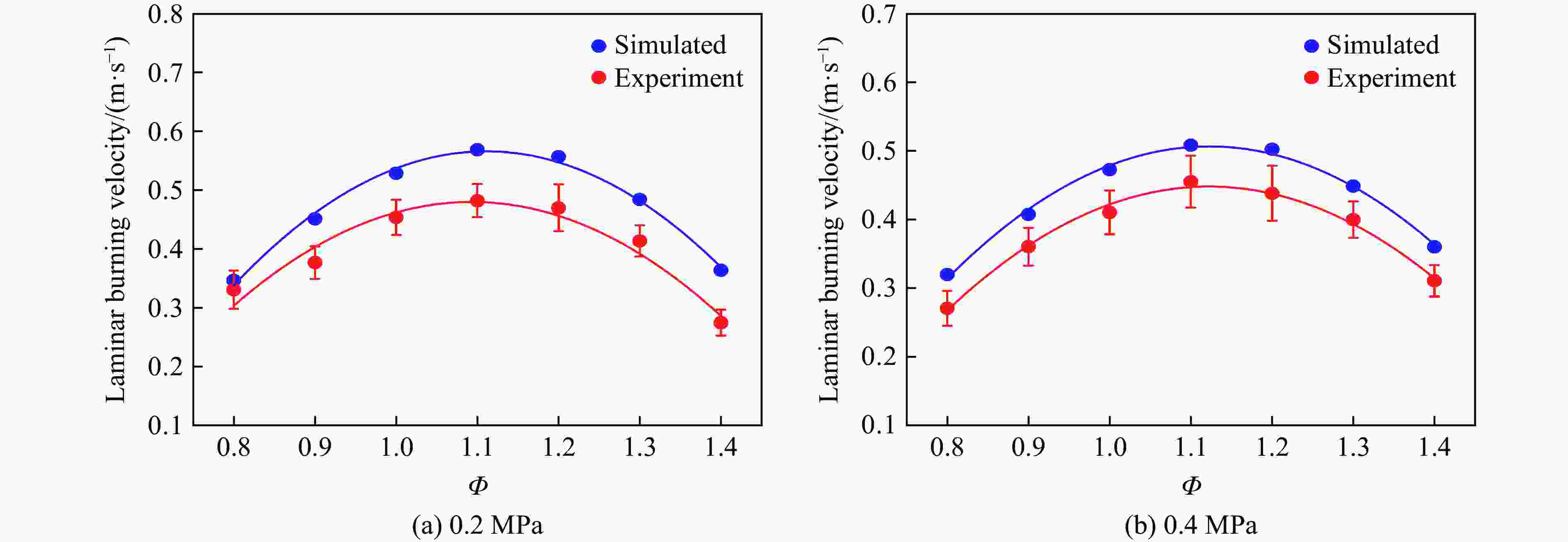
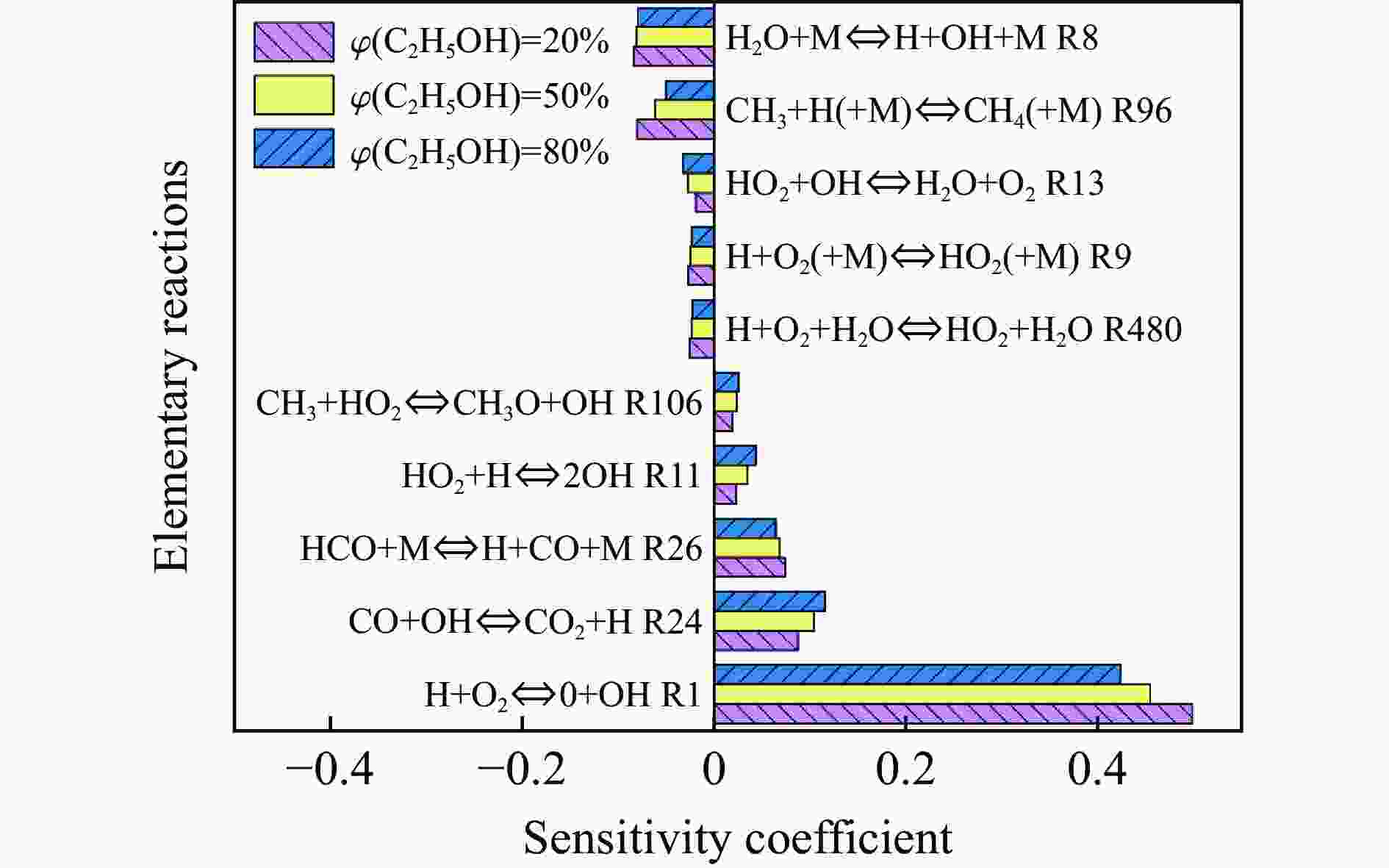
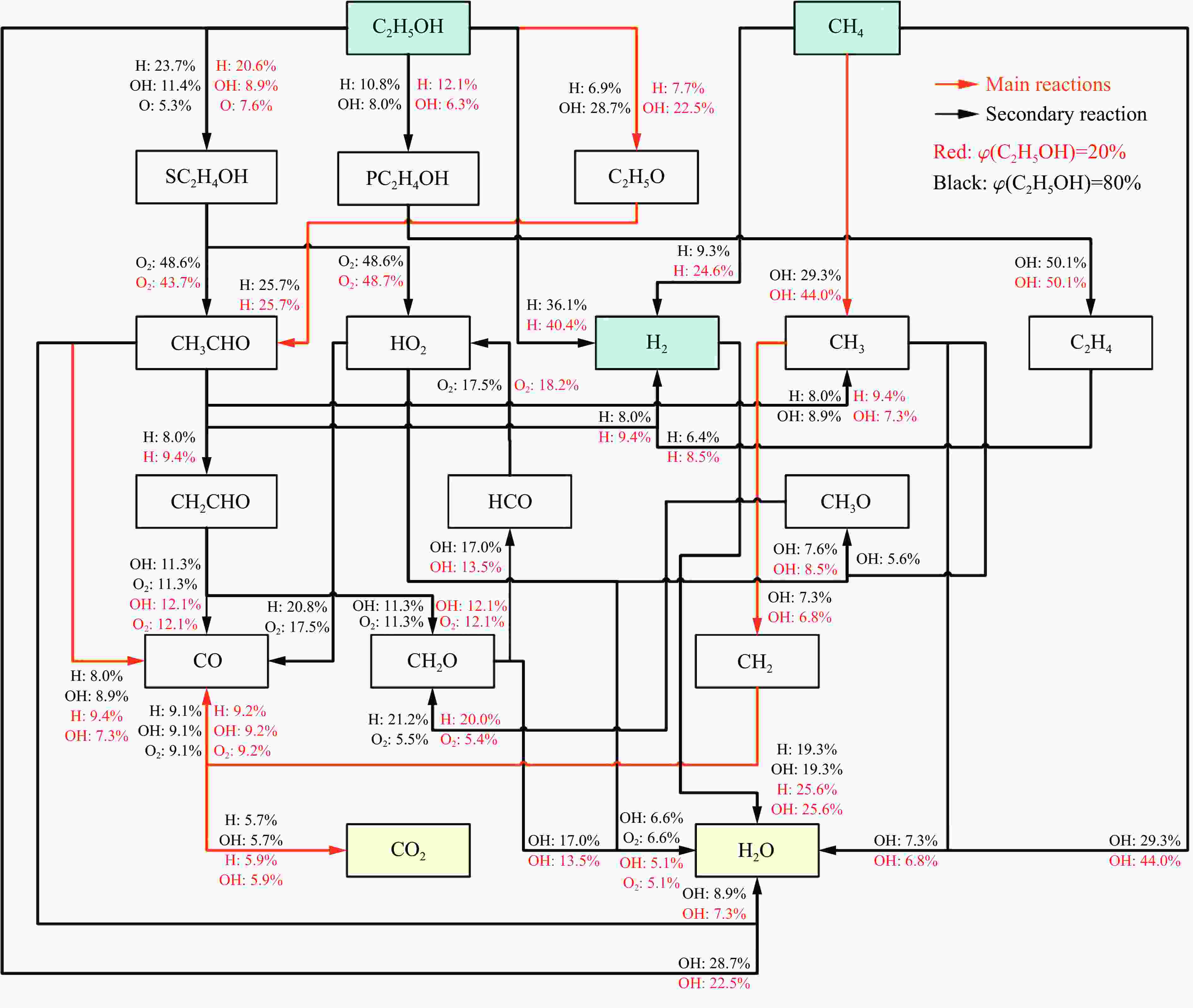
| [1] |
ALANNE K, CAO S L. An overview of the concept and technology of ubiquitous energy [J]. Applied Energy, 2019, 238: 284–302. DOI: 10.1016/j.apenergy.2019.01.100.
|
| [2] |
SUN X Y, LIU H L, DUAN X B, et al. Effect of hydrogen enrichment on the flame propagation, emissions formation and energy balance of the natural gas spark ignition engine [J]. Fuel, 2022, 307: 121843. DOI: 10.1016/j.fuel.2021.121843.
|
| [3] |
ORUC O, DINCER I. Assessing the potential of thermo-chemical water splitting cycles: a bridge towards clean and sustainable hydrogen generation [J]. Fuel, 2021, 286: 119325. DOI: 10.1016/j.fuel.2020.119325.
|
| [4] |
SHARMA Y C, KUMAR A, PRASAD R, et al. Ethanol steam reforming for hydrogen production: latest and effective catalyst modification strategies to minimize carbonaceous deactivation [J]. Renewable and Sustainable Energy Reviews, 2017, 74: 89–103. DOI: 10.1016/j.rser.2017.02.049.
|
| [5] |
ABDIN Z, ZAFARANLOO A, RAFIEE A, et al. Hydrogen as an energy vector [J]. Renewable and Sustainable Energy Reviews, 2020, 120: 109620. DOI: 10.1016/j.rser.2019.109620.
|
| [6] |
MA Y, WANG X R, LI T, et al. Hydrogen and ethanol: production, storage, and transportation [J]. International Journal of Hydrogen Energy, 2021, 46(54): 27330–27348. DOI: 10.1016/j.ijhydene.2021.06.027.
|
| [7] |
NYKYFORCHYN H, UNIGOVSKYI L, ZVIRKO O, et al. Pipeline durability and integrity issues at hydrogen transport via natural gas distribution network [J]. Procedia Structural Integrity, 2021, 33: 646–651. DOI: 10.1016/j.prostr.2021.10.071.
|
| [8] |
ZHANG Y, CAO W G, SHU C M, et al. Dynamic hazard evaluation of explosion severity for premixed hydrogen–air mixtures in a spherical pressure vessel [J]. Fuel, 2020, 261: 116433. DOI: 10.1016/j.fuel.2019.116433.
|
| [9] |
LI Q, CHENG Y, HUANG Z H. Comparative assessment of the explosion characteristics of alcohol-air mixtures [J]. Journal of Loss Prevention in the Process Industries, 2015, 37: 91–100. DOI: 10.1016/j.jlp.2015.07.003.
|
| [10] |
MITU M, BRANDES E. Influence of pressure, temperature and vessel volume on explosion characteristics of ethanol/air mixtures in closed spherical vessels [J]. Fuel, 2017, 203: 460–468. DOI: 10.1016/j.fuel.2017.04.124.
|
| [11] |
OPPONG F, XU C S, LUO Z Y, et al. Evaluation of explosion characteristics of 2-methylfuran/air mixture [J]. Journal of Loss Prevention in the Process Industries, 2019, 62: 103954. DOI: 10.1016/j.jlp.2019.103954.
|
| [12] |
LI Y C, BI M S, LI B, et al. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels [J]. Fuel, 2018, 233: 269–282. DOI: 10.1016/j.fuel.2018.06.042.
|
| [13] |
HU E J, TIAN H Z, ZHANG X Y, et al. Explosion characteristics of n-butanol/iso-octane-air mixtures [J]. Fuel, 2017, 188: 90–97. DOI: 10.1016/j.fuel.2016.10.002.
|
| [14] |
ZHANG B, NG H D. Explosion behavior of methane–dimethyl ether/air mixtures [J]. Fuel, 2015, 157: 56–63. DOI: 10.1016/j.fuel.2015.04.058.
|
| [15] |
ZHENG L G, ZHU X C, WANG Y L, et al. Combined effect of ignition position and equivalence ratio on the characteristics of premixed hydrogen/air deflagrations [J]. International Journal of Hydrogen Energy, 2018, 43(33): 16430–16441. DOI: 10.1016/j.ijhydene.2018.06.189.
|
| [16] |
时高龙, 温小萍, 王发辉, 等. 预混气体爆炸火焰与压力的耦合振荡特性 [J]. 化工学报, 2019, 70(7): 2811–2818. DOI: 10.11949/0438-1157.20190070.SHI G L, WEN X P, WANG F H, et al. Coupling oscillation characteristics of premixed gas explosion flame and pressure [J]. CIESC Journal, 2019, 70(7): 2811–2818. DOI: 10.11949/0438-1157.20190070.
|
| [17] |
余明高, 马梓茂, 韩世新, 等. 障碍物阻塞率梯度对甲烷爆炸特性影响研究 [J]. 化工学报, 2021, 72(10): 5430–5439. DOI: 10.11949/0438-1157.20210525.YU M G, MA Z M, HAN S X, et al. Study on influence of obstacle blockage rate gradient on methane explosion characteristics [J]. CIESC Journal, 2021, 72(10): 5430–5439. DOI: 10.11949/0438-1157.20210525.
|
| [18] |
SUN Z Y. Experimental studies on the explosion indices in turbulent stoichiometric H2/CH4/air mixtures [J]. International Journal of Hydrogen Energy, 2019, 44(1): 469–476. DOI: 10.1016/j.ijhydene.2018.02.094.
|
| [19] |
CAMMAROTA F, DI BENEDETTO A, DI SARLI V, et al. Combined effects of initial pressure and turbulence on explosions of hydrogen-enriched methane/air mixtures [J]. Journal of Loss Prevention in the Process Industries, 2009, 22(5): 607–613. DOI: 10.1016/j.jlp.2009.05.001.
|
| [20] |
CAMMAROTA F, DI BENEDETTO A, DI SARLI V, et al. The effect of hydrogen addition on the explosion of ethanol/air mixtures [J]. Chemical Engineering Transactions, 2012, 26: 405–410. DOI: 10.3303/CET1226068.
|
| [21] |
韦双明, 余明高, 裴蓓, 等. 三元混合气体燃料爆炸特性实验研究 [J]. 化工学报, 2022, 73(1): 451–460. DOI: 10.11949/0438-1157.20211256.WEI S M, YU M G, PEI B, et al. Experimental study on explosion characteristics of ternary mixed gas fuel [J]. CIESC Journal, 2022, 73(1): 451–460. DOI: 10.11949/0438-1157.20211256.
|
| [22] |
ZHOU Q, CHEUNG C S, LEUNG C W, et al. Effects of fuel composition and initial pressure on laminar flame speed of H2/CO/CH4 bio-syngas [J]. Fuel, 2019, 238: 149–158. DOI: 10.1016/j.fuel.2018.10.106.
|
| [23] |
WEI S M, YU M G, PEI B, et al. Effect of hydrogen enrichment on the laminar burning characteristics of dimethyl-ether/methane fuel: experimental and modeling study [J]. Fuel, 2021, 305: 121475. DOI: 10.1016/j.fuel.2021.121475.
|
| [24] |
FAGHIH M, GOU X L, CHEN Z. The explosion characteristics of methane, hydrogen and their mixtures: a computational study [J]. Journal of Loss Prevention in the Process Industries, 2016, 40: 131–138. DOI: 10.1016/j.jlp.2015.12.015.
|
| [25] |
ZHOU Q, CHEUNG C S, LEUNG C W, et al. Explosion characteristics of bio-syngas at various fuel compositions and dilutions in a confined vessel [J]. Fuel, 2020, 259: 116254. DOI: 10.1016/j.fuel.2019.116254.
|
| [26] |
ZHANG L, MA H H, PAN J, et al. Effects of hydrogen addition on the explosion characteristics of n-hexane/air mixtures [J]. International Journal of Hydrogen Energy, 2019, 44(3): 2029–2038. DOI: 10.1016/j.ijhydene.2018.11.085.
|
| [27] |
DAI T K, ZHANG B, LIU H. On the explosion characteristics for central and end-wall ignition in hydrogen-air mixtures: a comparative study [J]. International Journal of Hydrogen Energy, 2021, 46(60): 30861–30869. DOI: 10.1016/j.ijhydene.2021.06.213.
|
| [28] |
LIU Y, ZHANG Y G, ZHAO D F, et al. Effects of initial temperature and pressure on explosion characteristics of propane-diluent-air mixtures [J]. Journal of Loss Prevention in the Process Industries, 2021, 72: 104585. DOI: 10.1016/j.jlp.2021.104585.
|
| [29] |
National Fire Protection Association. Guide for venting of deflagrations: NFPA 68 [R]. USA: National Fire Protection Association, 2002.
|
| [30] |
CUI G, WANG S, LIU J G, et al. Explosion characteristics of a methane/air mixture at low initial temperatures [J]. Fuel, 2018, 234: 886–893. DOI: 10.1016/j.fuel.2018.07.139.
|
| [31] |
ZHANG B, XIU G L, BAI C H. Explosion characteristics of argon/nitrogen diluted natural gas-air mixtures [J]. Fuel, 2014, 124: 125–132. DOI: 10.1016/j.fuel.2014.01.090.
|
| [32] |
SHEN X B, ZHANG B, ZHANG X L, et al. Explosion characteristics of methane-ethane mixtures in air [J]. Journal of Loss Prevention in the Process Industries, 2017, 45: 102–107. DOI: 10.1016/j.jlp.2016.11.012.
|
| [33] |
LI Q Q, CHENG Y, JIN W, et al. Comparative study on the explosion characteristics of pentanol isomer-air mixtures [J]. Fuel, 2015, 161: 78–86. DOI: 10.1016/j.fuel.2015.08.027.
|
| [34] |
XU C S, WANG H Y, LI X L, et al. Explosion characteristics of a pyrolysis biofuel derived from rice husk [J]. Journal of Hazardous Materials, 2019, 369: 324–333. DOI: 10.1016/j.jhazmat.2019.01.101.
|
| [35] |
MITU M, GIURCAN V, RAZUS D, et al. Temperature and pressure influence on ethane-air deflagration parameters in a spherical closed vessel [J]. Energy Fuels, 2012, 26(8): 4840–4848. DOI: 10.1021/ef300849r.
|
| [36] |
DAHOE A E, DE GOEY L P H. On the determination of the laminar burning velocity from closed vessel gas explosions [J]. Journal of Loss Prevention in the Process Industries, 2003, 16(6): 457–478. DOI: 10.1016/S0950-4230(03)00073-1.
|
| [37] |
SHEN X B, XIU G L, WU S Z. Experimental study on the explosion characteristics of methane/air mixtures with hydrogen addition [J]. Applied Thermal Engineering, 2017, 120: 741–747. DOI: 10.1016/j.applthermaleng.2017.04.040.
|
| [38] |
WANG X, FU J Q, XIE M K, et al. Numerical investigation of laminar burning velocity for methane-hydrogen-air mixtures at wider boundary conditions [J]. Aerospace Science and Technology, 2022, 121: 107393. DOI: 10.1016/j.ast.2022.107393.
|
| [39] |
MARINOV N M. A detailed chemical kinetic model for high temperature ethanol oxidation [J]. International Journal of Chemical Kinetics, 1999, 31(3): 183–220. DOI: 10.1002/(SICI)1097-4601(1999)31:3<183::AID-KIN3>3.0.CO;2-X.
|
| [40] |
XIANG L K, JIANG H T, REN F, et al. Numerical study of the physical and chemical effects of hydrogen addition on laminar premixed combustion characteristics of methane and ethane [J]. International Journal of Hydrogen Energy, 2020, 45(39): 20501–20514. DOI: 10.1016/j.ijhydene.2019.11.040.
|
| [41] |
ECKART S, PIZZUTI L, FRITSCHE C, et al. Experimental study and proposed power correlation for laminar burning velocity of hydrogen-diluted methane with respect to pressure and temperature variation [J]. International Journal of Hydrogen Energy, 2022, 47(9): 6334–6348. DOI: 10.1016/j.ijhydene.2021.11.243.
|
| [42] |
OPPONG F, XU C S, LUO Z Y, et al. Investigating the explosion of ethyl acetate in the presence of hydrogen [J]. International Journal of Hydrogen Energy, 2020, 45(39): 20400–20407. DOI: 10.1016/j.ijhydene.2019.12.136.
|
| [43] |
周康泉. 容弹上用定容法测量层流燃烧速度的研究 [D]. 杭州: 浙江大学, 2019: 9–11.ZHOU K Q. The research of measuring the laminar buring velocity using CVM in the constant volume burning vessel [D]. Hangzhou: Zhejiang University, 2019: 9–11.
|






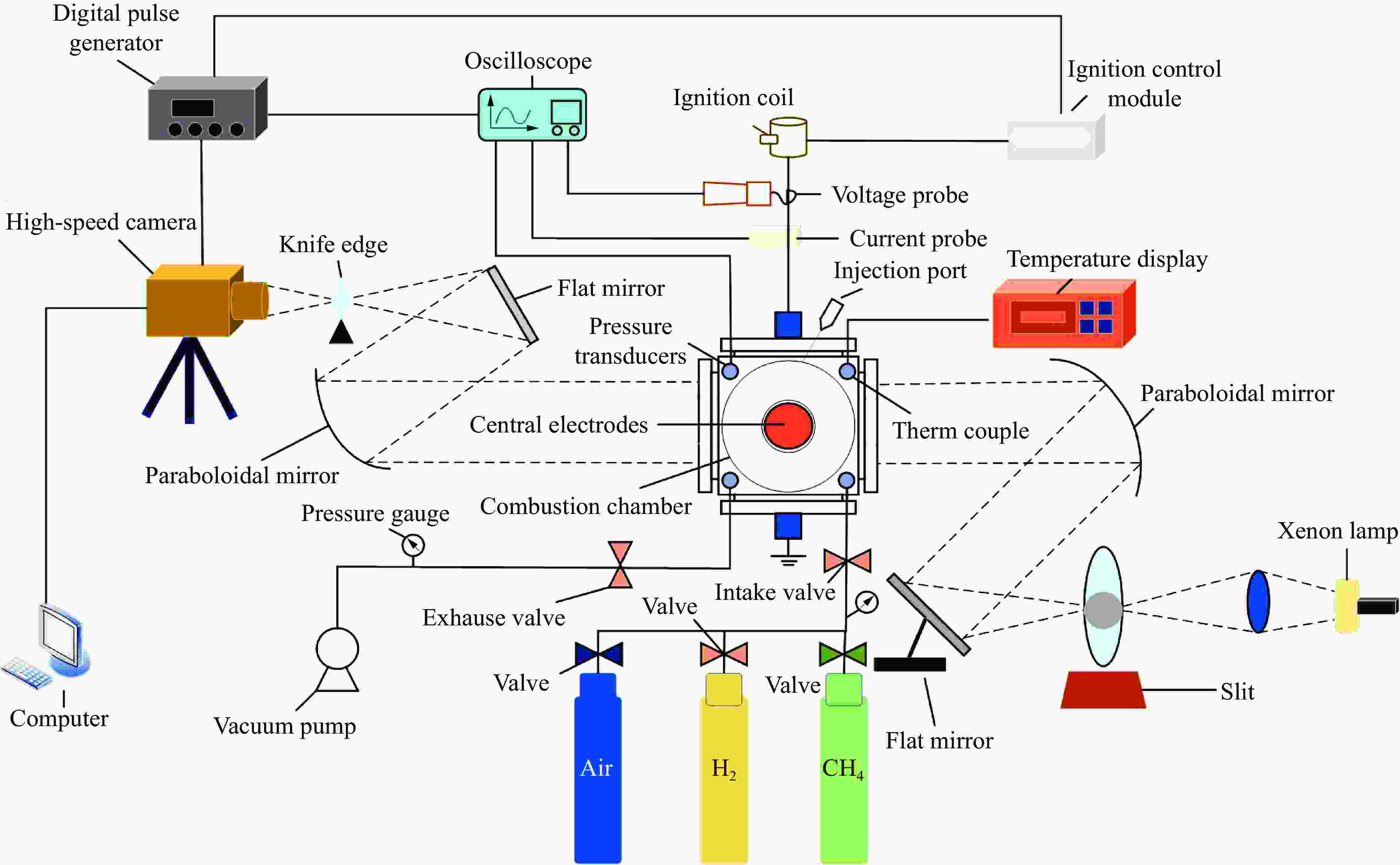
 下载:
下载: