Experimental study on methane-air mixtures explosion limits at normal and elevated initial temperatures and pressures
-
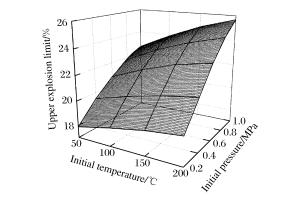
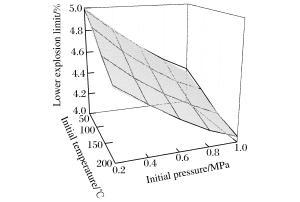
摘要: 为了研究不同初始条件对甲烷-空气混合物爆炸极限的影响,利用容积为20 L的爆炸罐,在不同初始温度(25~200 ℃)和初始压力(0.1~1.0 MPa)条件下测定了甲烷-空气混合物的爆炸极限。实验结果表明,随着初始温度和初始压力的升高,爆炸上限升高,爆炸下限降低,爆炸极限范围扩大。在实验温度和压力范围内,常压/常温条件下,爆炸上限和下限与初始温度/初始压力呈线性相关。爆炸上限与初始温度的相关性受初始压力的影响,其与初始压力的相关性也与初始温度有关。然而,初始压力/初始温度对爆炸下限的影响与初始温度/初始压力的相关性并不显著。初始温度和初始压力对爆炸极限的耦合影响比单一因素对其的影响大,且相较而言,其对爆炸上限的影响更为显著。本文中绘制了影响曲面来描述初始温度和初始压力如何影响甲烷-空气混合物的爆炸极限。Abstract: In order to study the influence of initial conditions on methane-air mixtures explosion limits, the explosion limits of methane-air mixtures were obtained experimentally at different initial temperatures up to 200 ℃ and initial pressures up to 1.0 MPa. The experiments were performed in a closed spherical 20 dm3 vessel with an ignition electrode at the center. The results show that with the increasing of initial temperature and initial pressure, the upper explosion limit increases, but the lower explosion limit decreases, that is the explosion limit expands. At atmospheric pressure/ambient temperature, the dependences of the upper explosion limit and lower explosion limit on initial temperature and initial pressure are both linear in the experimental temperature-pressure ranges. The dependence of the upper explosion limit on initial temperature/initial pressure is influenced by the initial pressure/initial temperature, but the dependence of the lower explosion limit on those is not influenced obviously. The coupling effects of initial temperature and initial pressure on the upper explosion limit and lower explosion limit are greater than that of a single factor, especially on the upper explosion limit. Surfaces are formed to describe how the initial temperature and initial pressure influence the upper explosion limit and the lower explosion limit of methane-air mixtures.
-
Key words:
- explosion limits /
- initial pressure /
- initial temperature /
- methane-air mixtures
-
表 1 拟合函数的参数
Table 1. Parameters for fitting function
y% x A B R UEL T0/℃ 15.464 3 0.011 4 0.995 5 UEL p0/MPa 15.659 7 6.142 4 0.987 6 LEL T0/℃ 5.142 9 -0.002 5 -0.996 6 LEL p0/MPa 5.151 2 -0.666 9 -0.998 5 表 2 拟合函数的参数
Table 2. Parameters for fitting function
z x y z0 A UEL/% T0/℃ p0/MPa 13.80 12.30 xc w1 yc w2 R2 263.81 267.86 1.13 0.75 0.998 9 表 3 拟合函数的参数
Table 3. Parameters for fitting function
z x y z0 LEL/% T0/℃ p0/MPa 3.08 A B C R2 2.34 587.71 1.81 0.995 3 -
[1] Coronado C J, Carvalho J A Jr, Andrade J C, et al.Flammability limits:A review with emphasis on ethanol for aeronautical applications and description of the experimental procedure[J].Journal of Hazardous Materials, 2012, 241-242:32-54. doi: 10.1016/j.jhazmat.2012.09.035 [2] Van den Schoor F, Verplaetsen F, Berghmans J.Calculation of the upper flammability limit of methane/hydrogen/air mixtures at elevated pressures and temperature[J].International Journal of Hydrogen Energy, 2008, 33(4):1399-1406. doi: 10.1016/j.ijhydene.2008.01.002 [3] Van den Schoor F, Verplaetsen F, Berghmans J.Calculation of the upper flammability limit of methane/air mixtures at elevated pressures and temperatures[J].Journal of Hazardous Materials, 2008, 153(3):1301-1307. doi: 10.1016/j.jhazmat.2007.09.088 [4] Van den Schoor F, Hermanns R TE, Van Oijen J A, et al.Comparison and evaluation of methods for the determination of flammability limits, applied to methane/hydrogen/air mixtures[J].Journal of Hazardous Materials, 2008, 150(3):573-581. doi: 10.1016/j.jhazmat.2007.05.006 [5] Coward H F, Jones G W.Limits of flammability of gases and vapors[M].Washington:United States Government Printing Office, 1952:37-41. [6] Zabetakis M G.Flammability characteristics of combustible gases and vapors[M].Washington:United States Government Printing Office, 1965:20-27. [7] Cashdollar K L, Zlochower I A, Green G M, et al.Flammability of methane, propane, and hydrogen gases[J].Journal of Loss Prevention in the Process Industries, 2000, 13(3/4/5):327-340. http://www.sciencedirect.com/science/article/pii/S0950423099000376 [8] Wierzba I, Ale B B.Rich flammability limits of fuel mixture involving hydrogen at elevated temperature[J].International Journal of Hydrogen Energy, 2000, 25(1):75-80. doi: 10.1016/S0360-3199(99)00009-9 [9] Gieras M, Klemens R, Rarata G, et al.Determination of explosion parameters of methane-air mixtures in the chamber of 40 dm3 at normal and elevated temperature[J].Journal of Loss Prevention in the Process Industries, 2006, 19(2/3):263-270. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d4c91882e6c30ee838be2c12bc6d4931 [10] Cooper C M, Wiezevich P J.Effects of temperature and pressure on the upper explosion limit of methane-oxygen mixtures[J].Journal of Industrial and Engineering Chemistry, 1929, 21(12):1210-1214. doi: 10.1021/ie50240a014 [11] Vanderstraeten B, Tuerlinckx D, Berghmans J, et al.Experimental study of the pressure and temperature dependence on the upper flammability limit of methane/air mixtures[J].Journal of Hazardous Materials, 1997, 56(3):237-246. doi: 10.1016/S0304-3894(97)00045-9 [12] Van den Schoor F, Verplaetsen F.The upper explosion limit of lower alkanes and alkenes in air at elevated pressure and temperature[J].Journal of Hazardous Materials, 2006, 128(1):1-9. doi: 10.1016/j.jhazmat.2005.06.043 [13] Van den Schoor F, Verplaetsen F.The upper flammability limit of methane/hydrogen/air mixtures at elevated pressure and temperatures[J].International Journal of Hydrogen Energy, 2008, 32(13):2548-2552. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d29ed8e4047b8942cbe024fb139ff2b9 [14] ASTM International.Standard test methods for limiting oxygen (oxidant) concentration in gases and vapors:ASTM E2079-07[S].American Society for Testing and Materials, 2013:1-2. -






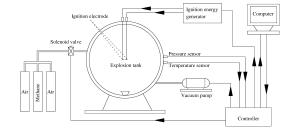
 下载:
下载: