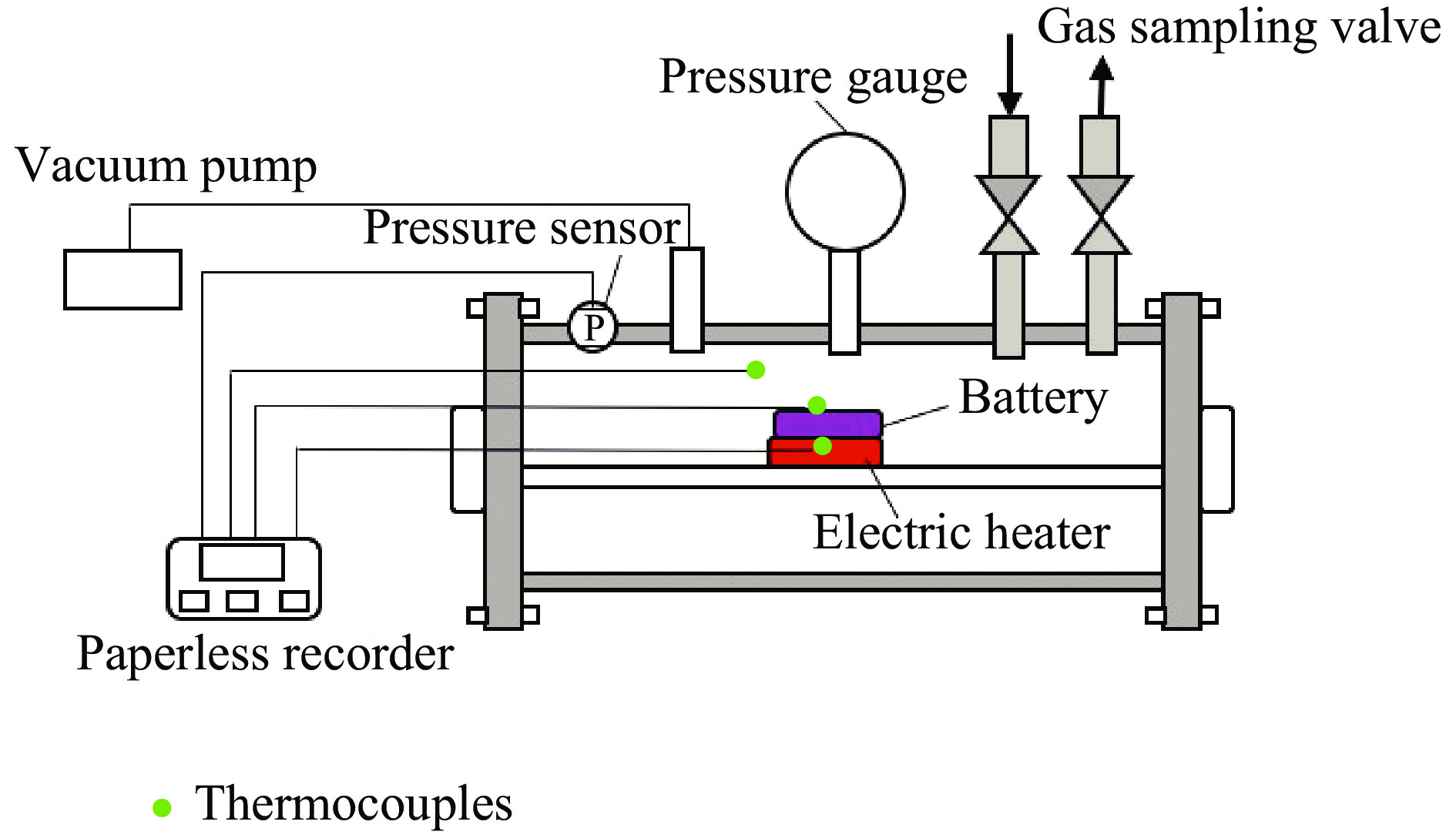
Prediction methods for lower explosion limit of thermal runaway products of lithium-iron phosphate batteries
-
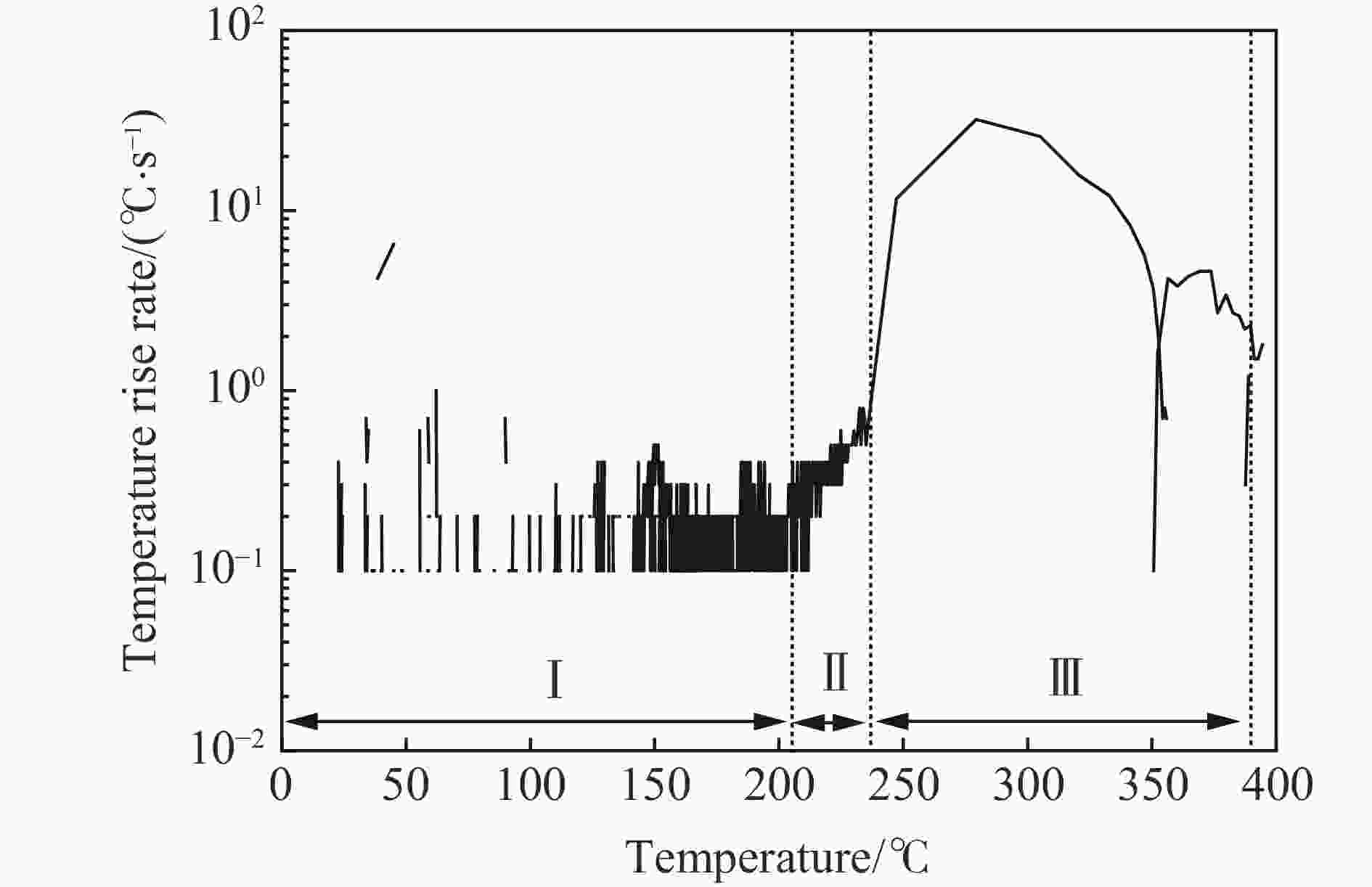
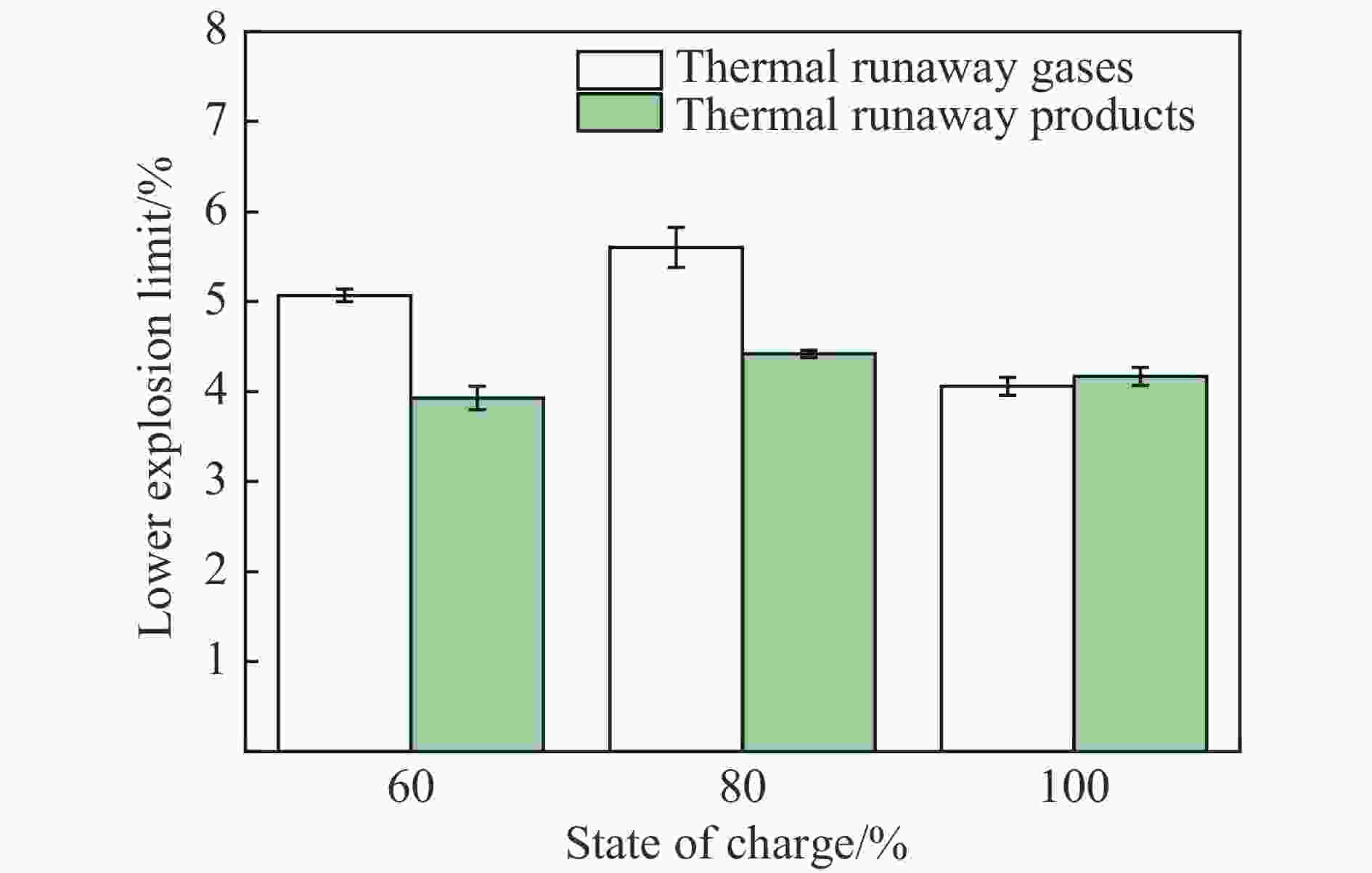
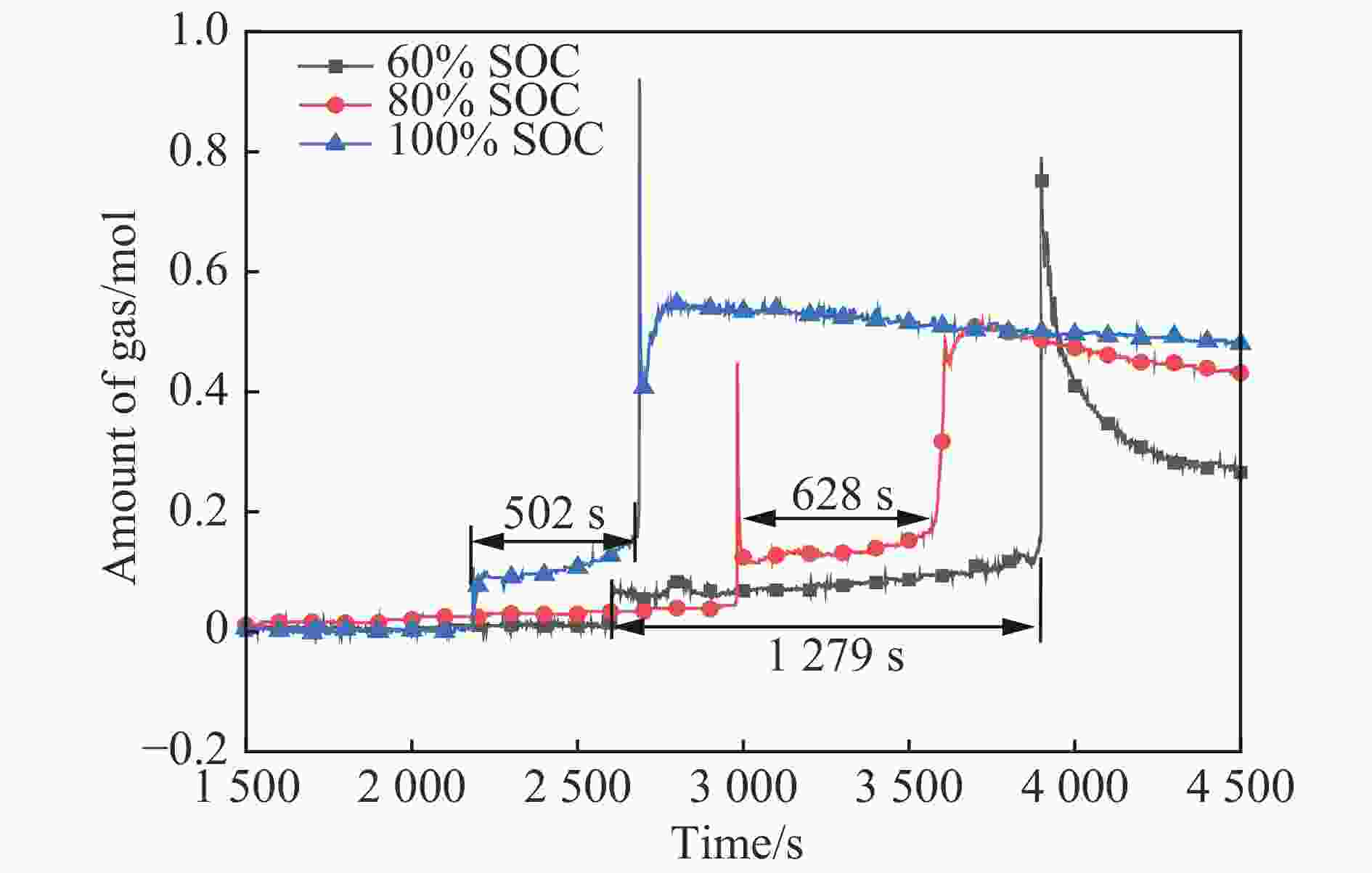
摘要: 为准确预测磷酸铁锂电池热失控产物的爆炸下限,在密闭压力容器内开展了磷酸铁锂电池热失控实验,结合热失控特性和气相色谱-质谱联用技术,计算了热失控产物气体组分,基于能量守恒方程和绝热火焰温度,建立了磷酸铁锂电池热失控产物爆炸下限的预测模型,并验证了绝热火焰温度法、Le Chatelier法和Jones法的准确性,考察了电解液蒸气对热失控产物爆炸下限的影响。结果表明,常温下采用Le Chatelier法计算得到的爆炸下限偏差最小,为1.14%;采用绝热火焰温度法计算结果偏差最大,为10.02%。在60%~100%荷电状态(state of charge, SOC)范围内,磷酸铁锂电池热失控气体的爆炸下限先升后降。当热失控产物考虑电解液蒸气时,60% SOC磷酸铁锂电池热失控产物爆炸下限仅为3.93%,较未考虑电解液蒸气热失控气体的爆炸下限降低了22.49%,这说明电解液蒸气提高了磷酸铁锂电池热失控产物的爆炸风险。
-
关键词:
- 磷酸铁锂电池 /
- 热失控产物 /
- 爆炸下限 /
- Le Chatelier定律 /
- 绝热火焰温度
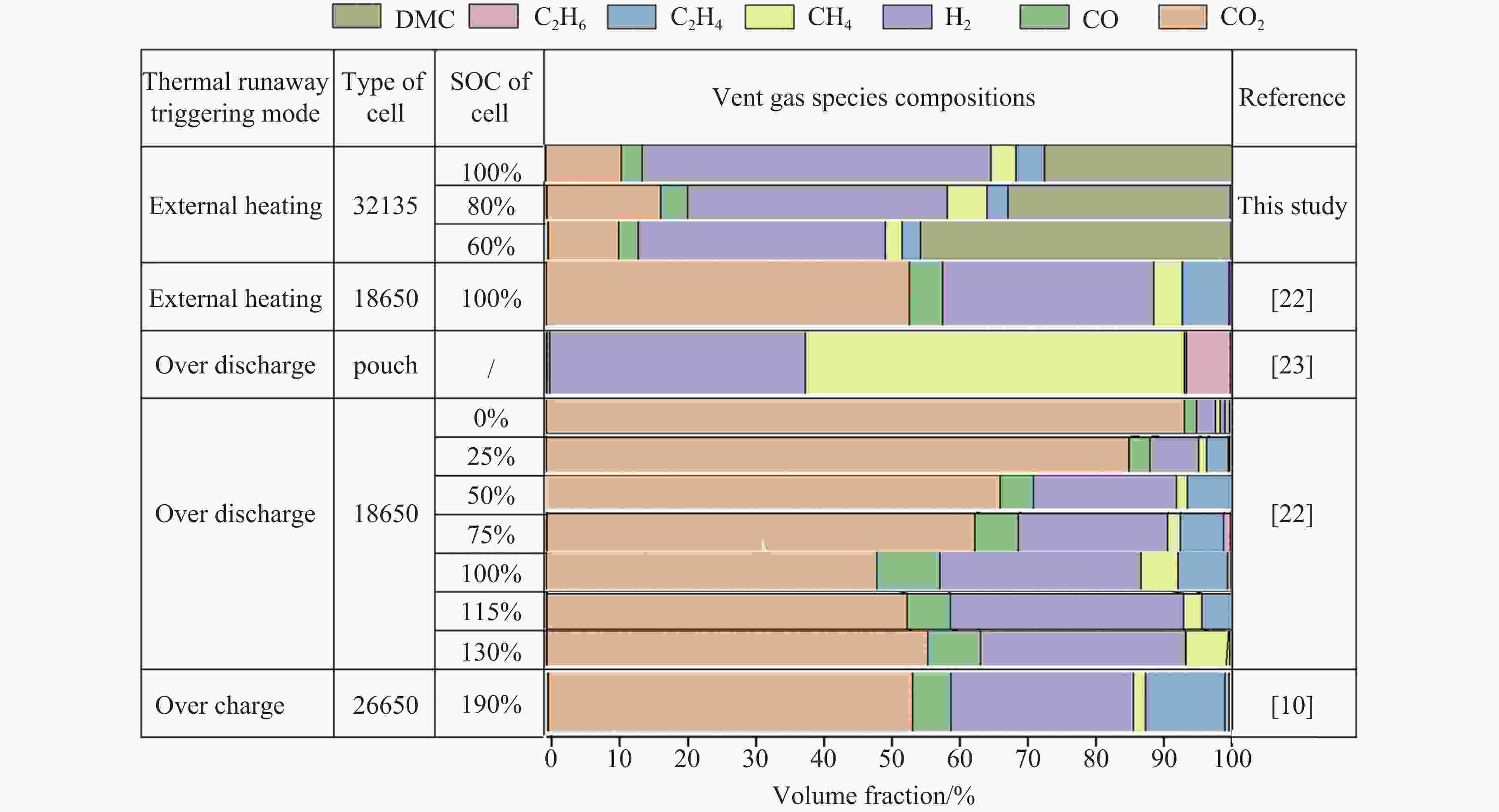
Abstract: To predict precisely the lower explosion limit of thermal runaway products of lithium iron phosphate batteries, thermal runaway tests of lithium iron phosphate batteries were carried out in a closed pressure vessel. The experiments were carried out at 25 ℃ and 0.1 MPa, and the method was used to analyze the thermal runaway gas production. The vent gas species composition of lithium iron phosphate batteries was analyzed by gas chromatography and mass spectrometry. Combined with the thermal runaway characteristics of the battery and gas chromatography-mass spectrometry (GC-MS) technology, the gas composition of thermal runaway products of lithium iron phosphate batteries was calculated. It was assumed that the thermal runway products released from the relief valve to the first injection were all dimethyl carbonate (DMC), and the secondary injection gas was the mixed gas generated by the internal chemical reaction, which is mainly composed of H2, CO2, CO, CH4, and C2H4. A prediction model of the lower explosion limit of thermal runaway products was established based on the energy conservation equation and adiabatic flame temperature. The prediction methods of lower explosion limit of multicomponent gases based on adiabatic flame temperature, Le Chatelier law method, and Jones method were verified, and the influence of electrolyte vapor on the lower explosion limit of thermal runaway production was also investigated. The smallest deviation of the lower explosion limit calculated by the Le Chatelier law method at normal temperature and pressure was 1.14%, and the largest deviation of the lower explosion limit calculated by the adiabatic flame temperature method was 10.02%. Within the range from 60% SOC to 100% SOC, the lower explosion limit of the thermal runaway gases increases first and then decreases. When the electrolyte vapor is considered in the thermal runaway products, the lower explosion limit of thermal runaway products of lithium iron phosphate batteries with 60% SOC is only 3.93%, which is 22.49% lower than that of the thermal runaway gas without considering the electrolyte vapor. Actually, the electrolyte vapor is contained in the thermal runaway products of lithium iron phosphate batteries. These results indicate that the addition of electrolyte vapor increases the explosion risk of thermal runaway production of lithium iron phosphate batteries. -
表 1 热失控产物各组分在爆炸下限处的绝热火焰温度
Table 1. Adiabatic flame temperature of each component of the thermal runaway products at their lower explosion limit
环境温度/℃ 绝热火焰温度/℃ H2 CO CH4 C2H4 DMC 60% SOC下热失控产物 80% SOC下热失控产物 100% SOC下热失控产物 25 354.0 1118.4 1207.3 1096.8 1161.0 833.1 792.1 698.6 50 372.5 1144.7 1208.8 1099.4 1163.0 842.5 802.8 710.9 75 363.9 1124.0 1211.0 1102.4 1168.0 841.1 800.1 707.0 100 408.4 1126.5 1211.7 1105.7 1171.0 860.7 821.8 733.5 -
[1] 高飞, 朱艳丽, 齐创, 等. 锂离子电池安全事故激源浅析 [J]. 电源技术, 2019, 43(3): 453–457. DOI: 10.3969/j.issn.1002-087X.2019.03.029.GAO F, ZHU Y L, QI C, et al. Excitation source analysis of lithium ion batteries safety accidents [J]. Chinese Journal of Power Sources, 2019, 43(3): 453–457. DOI: 10.3969/j.issn.1002-087X.2019.03.029. [2] CHEN S C, WANG Z R, WANG J H, et al. Lower explosion limit of the vented gases from Li-ion batteries thermal runaway in high temperature condition [J]. Journal of Loss Prevention in the Process Industries, 2020, 63: 103992. DOI: 10.1016/j.jlp.2019.103992. [3] 张伟, 郝朝龙, 刘添添, 等. 航空压力环境对锂离子电池热解气体爆炸极限影响 [J]. 中国安全生产科学技术, 2022, 18(11): 155–162. DOI: 10.11731/j.issn.1673-193x.2022.11.022.ZHANG W, HAO C L, LIU T T, et al. Influence of aviation pressure environment on explosion limit of pyrolysis gas from lithium-ion batteries [J]. Journal of Safety Science and Technology, 2022, 18(11): 155–162. DOI: 10.11731/j.issn.1673-193x.2022.11.022. [4] 郭超超, 张青松. 锂离子电池热解气体爆炸极限测定及其危险性分析 [J]. 中国安全生产科学技术, 2016, 12(9): 46–49. DOI: 10.11731/j.issn.1673-193x.2016.09.008.GUO C C, ZHANG Q S. Determination on explosion limit of pyrolysis gas released by lithium-ion battery and its risk analysis [J]. Journal of Safety Science and Technology, 2016, 12(9): 46–49. DOI: 10.11731/j.issn.1673-193x.2016.09.008. [5] LI W F, WANG H W, ZHANG Y J, et al. Flammability characteristics of the battery vent gas: a case of NCA and LFP lithium-ion batteries during external heating abuse [J]. Journal of Energy Storage, 2019, 24: 100775. DOI: 10.1016/j.est.2019.100775. [6] WANG H B, XU H, ZHANG Z L, et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: a comparative study [J]. eTransportation, 2022, 13: 100190. DOI: 10.1016/j.etran.2022.100190. [7] BAIRD A R, ARCHIBALD E J, MARR K C, et al. Explosion hazards from lithium-ion battery vent gas [J]. Journal of Power Sources, 2020, 446: 227257. DOI: 10.1016/j.jpowsour.2019.227257. [8] JONES G W. Inflammability of mixed gases: technical paper 450 [R]. Washington: United States Covernment Printing Office, 1929. [9] HENRIKSEN M, VAAGSAETHER K, LUNDBERG J, et al. Laminar burning velocity of gases vented from failed Li-ion batteries [J]. Journal of Power Sources, 2021, 506: 230141. DOI: 10.1016/j.jpowsour.2021.230141. [10] FERNANDES Y, BRY A, DE PERSIS S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery [J]. Journal of Power Sources, 2018, 389: 106–119. DOI: 10.1016/j.jpowsour.2018.03.034. [11] LARSSON F, BERTILSSON S, FURLANI M, et al. Gas explosions and thermal runaways during external heating abuse of commercial lithium-ion graphite-LiCoO2 cells at different levels of ageing [J]. Journal of Power Sources, 2018, 373: 220–231. DOI: 10.1016/j.jpowsour.2017.10.085. [12] FAN R J, WANG Z R, LU Y W, et al. Numerical analysis on the combustion characteristic of lithium-ion battery vent gases and the suppression effect [J]. Fuel, 2022, 330: 125450. DOI: 10.1016/j.fuel.2022.125450. [13] ZHANG Q S, LIU T T, HAO C L, et al. In situ Raman investigation on gas components and explosion risk of thermal runaway emission from lithium-ion battery [J]. Journal of Energy Storage, 2022, 56: 105905. DOI: 10.1016/j.est.2022.105905. [14] 杨娟, 牛江昊, 张青松. 循环老化对锂离子电池热失控气体爆炸危险性影响实验研究 [J]. 航空学报, 2024, 45(3): 428529. DOI: 10.7527/S1000-6893.2023.28529.YANG J, NIU J H, ZHANG Q S. Experimental research on the effect of cyclic aging on the detonation risk of thermal runaway gas explosion in lithium-ion batteries [J]. Acta Aeronautica et Astronautica Sinica, 2024, 45(3): 428529. DOI: 10.7527/S1000-6893.2023.28529. [15] LIU P J, LIU C Q, YANG K, et al. Thermal runaway and fire behaviors of lithium iron phosphate battery induced by over heating [J]. Journal of Energy Storage, 2020, 31: 101714. DOI: 10.1016/j.est.2020.101714. [16] LIU X, REN D S, HSU H, et al. Thermal runaway of lithium-ion batteries without internal short circuit [J]. Joule, 2018, 2(10): 2047–2064. DOI: 10.1016/j.joule.2018.06.015. [17] 张弦, 霍怡廷, 李宇, 等. 一种近理想气体状态方程及其热力学性质计算 [J]. 当代化工研究, 2020(13): 19–22. DOI: 10.3969/j.issn.1672-8114.2020.13.009.ZHANG X, HUO Y T, LI Y, et al. Calculation of thermodynamic properties for a near-ideal gas state equation [J]. Modern Chemical Research, 2020(13): 19–22. DOI: 10.3969/j.issn.1672-8114.2020.13.009. [18] GOLUBKOV A W, SCHEIKL S, PLANTEU R, et al. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes-impact of state of charge and overcharge [J]. RSC Advances, 2015, 5(70): 57171–57186. DOI: 10.1039/C5RA05897J. [19] KOCH S, FILL A, BIRKE K P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway [J]. Journal of Power Sources, 2018, 398: 106–112. DOI: 10.1016/j.jpowsour.2018.07.051. [20] ZHANG L, DUAN Q L, MENG X D, et al. Experimental investigation on intermittent spray cooling and toxic hazards of lithium-ion battery thermal runaway [J]. Energy Conversion and Management, 2022, 252: 115091. DOI: 10.1016/j.enconman.2021.115091. [21] COMAN P T, RAYMAN S, WHITE R E. A lumped model of venting during thermal runaway in a cylindrical lithium cobalt oxide lithium-ion cell [J]. Journal of Power Sources, 2016, 307: 56–62. DOI: 10.1016/j.jpowsour.2015.12.088. [22] GOLUBKOV A W, FUCHS D, WAGNER J, et al. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes [J]. RSC Advances, 2014, 4(7): 3633–3642. DOI: 10.1039/C3RA45748F. [23] ZHENG Y, QIAN K, LUO D, et al. Influence of over-discharge on the lifetime and performance of LiFePO4/graphite batteries [J]. RSC Advances, 2016, 6(36): 30474–30483. DOI: 10.1039/C6RA01677D. [24] 夏阳光, 陶刚, 张礼敬. 基于绝热火焰温度多元混合气体可燃性极限的理论预测 [J]. 中国安全生产科学技术, 2016, 12(9): 30–35. DOI: 10.11731/j.issn.1673-193x.2016.09.005.XIA Y G, TAO G, ZHANG L J. Theoretical prediction on flammable limit of multi-component gas mixture based on adiabatic flame temperature [J]. Journal of Safety Science and Technology, 2016, 12(9): 30–35. DOI: 10.11731/j.issn.1673-193x.2016.09.005. [25] 李国梁, 蒋军成, 潘勇. 基于绝热火焰温度混合气体爆炸下限的预测 [J]. 中国安全科学学报, 2011, 21(7): 57–61. DOI: 10.16265/j.cnki.issn1003-3033.2011.07.028.LI G L, JIANG J C, PAN Y. Prediction on lower explosive limit of mixed gases based on calculated adiabatic flame temperatures [J]. China Safety Science Journal, 2011, 21(7): 57–61. DOI: 10.16265/j.cnki.issn1003-3033.2011.07.028. [26] SHANG R X, LI G, WANG Z, et al. Experimental study on the lower flammability limit of H2/CO/air mixtures with N2 and CO2 dilution at elevated temperatures [J]. International Journal of Hydrogen Energy, 2020, 45(16): 10164–10175. DOI: 10.1016/j.ijhydene.2020.01.247. -






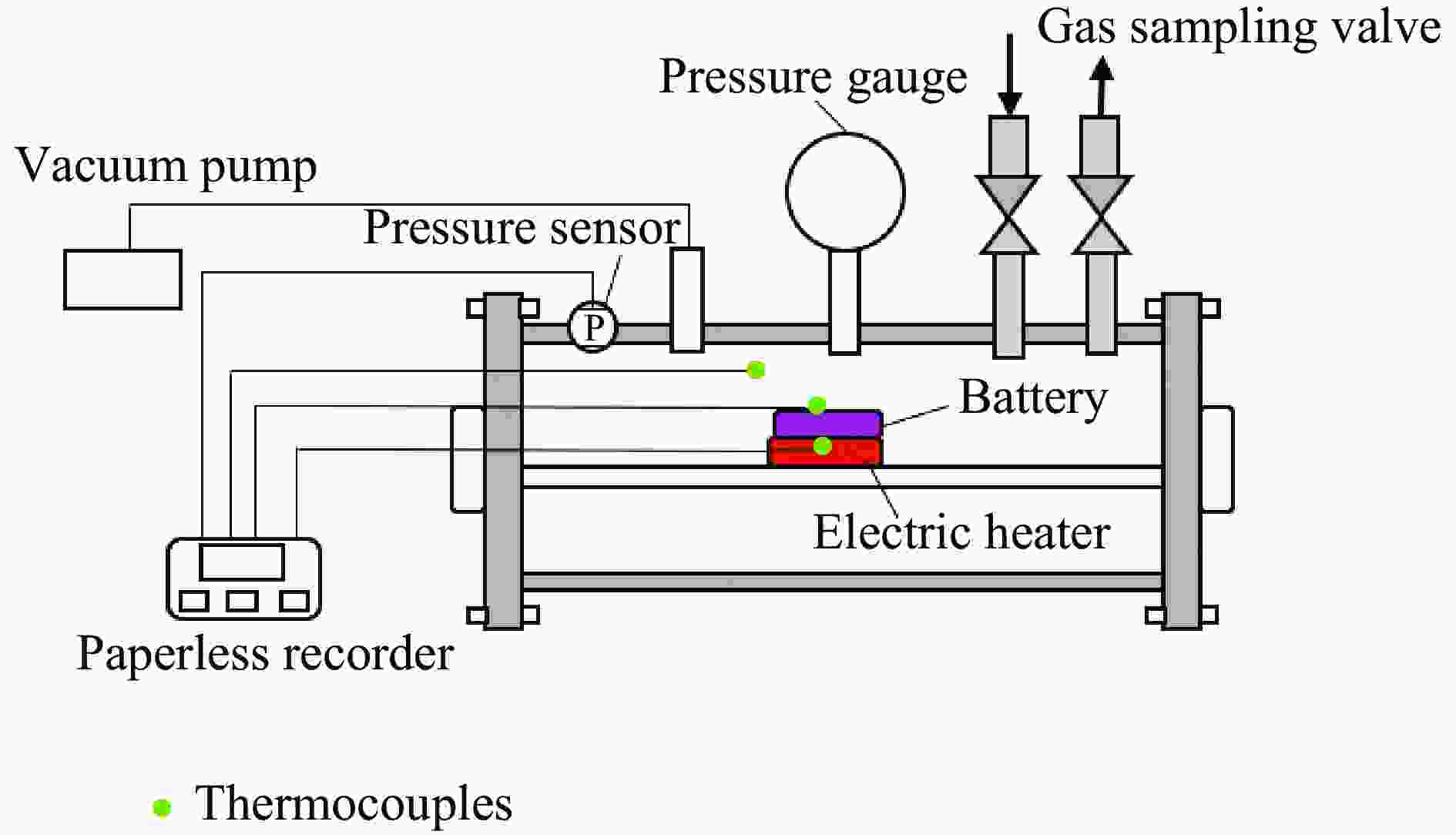
 下载:
下载: