Research progress on the pathogenesis and biomarkers of blast-induced traumatic brain injury
-
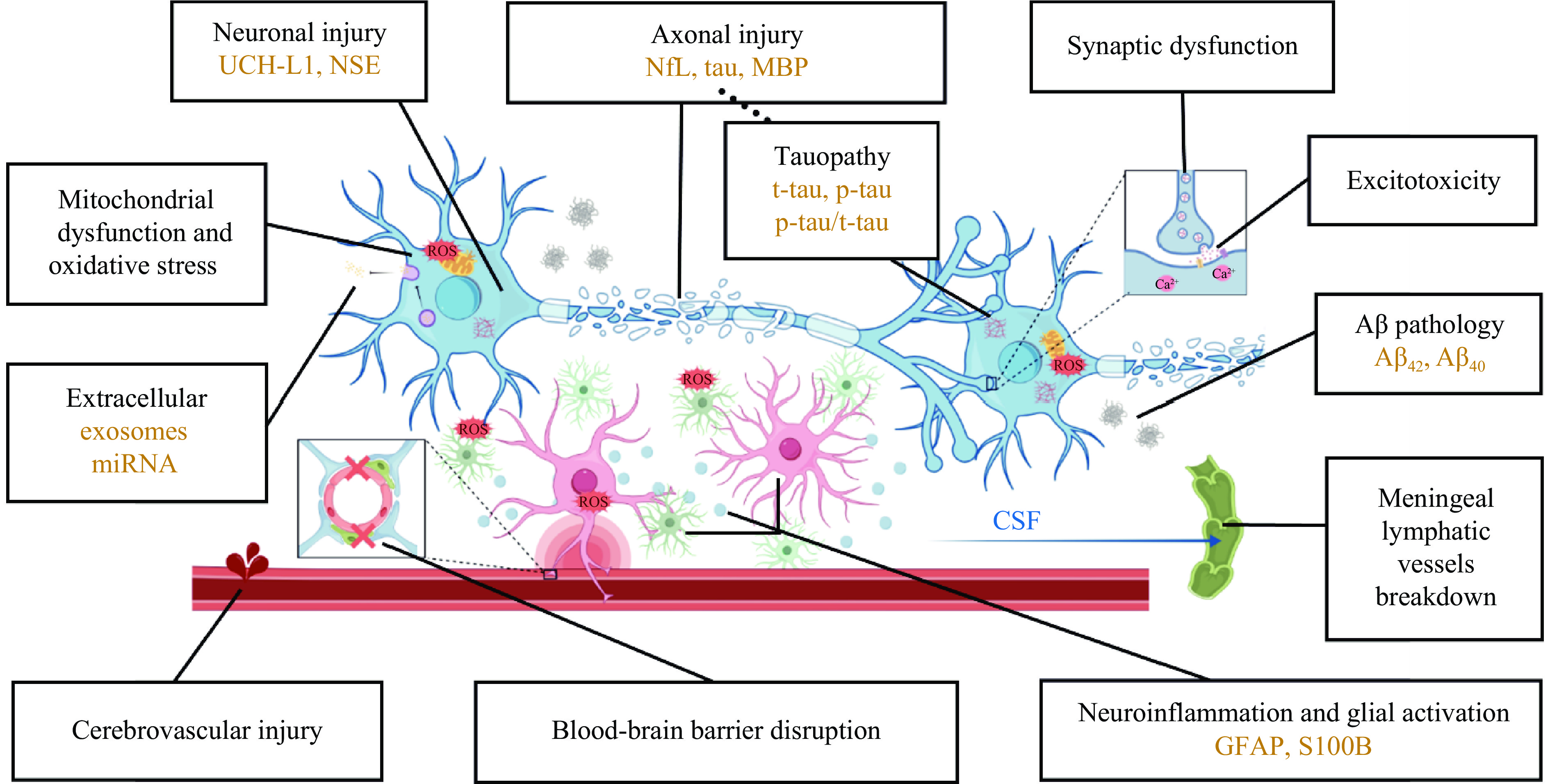
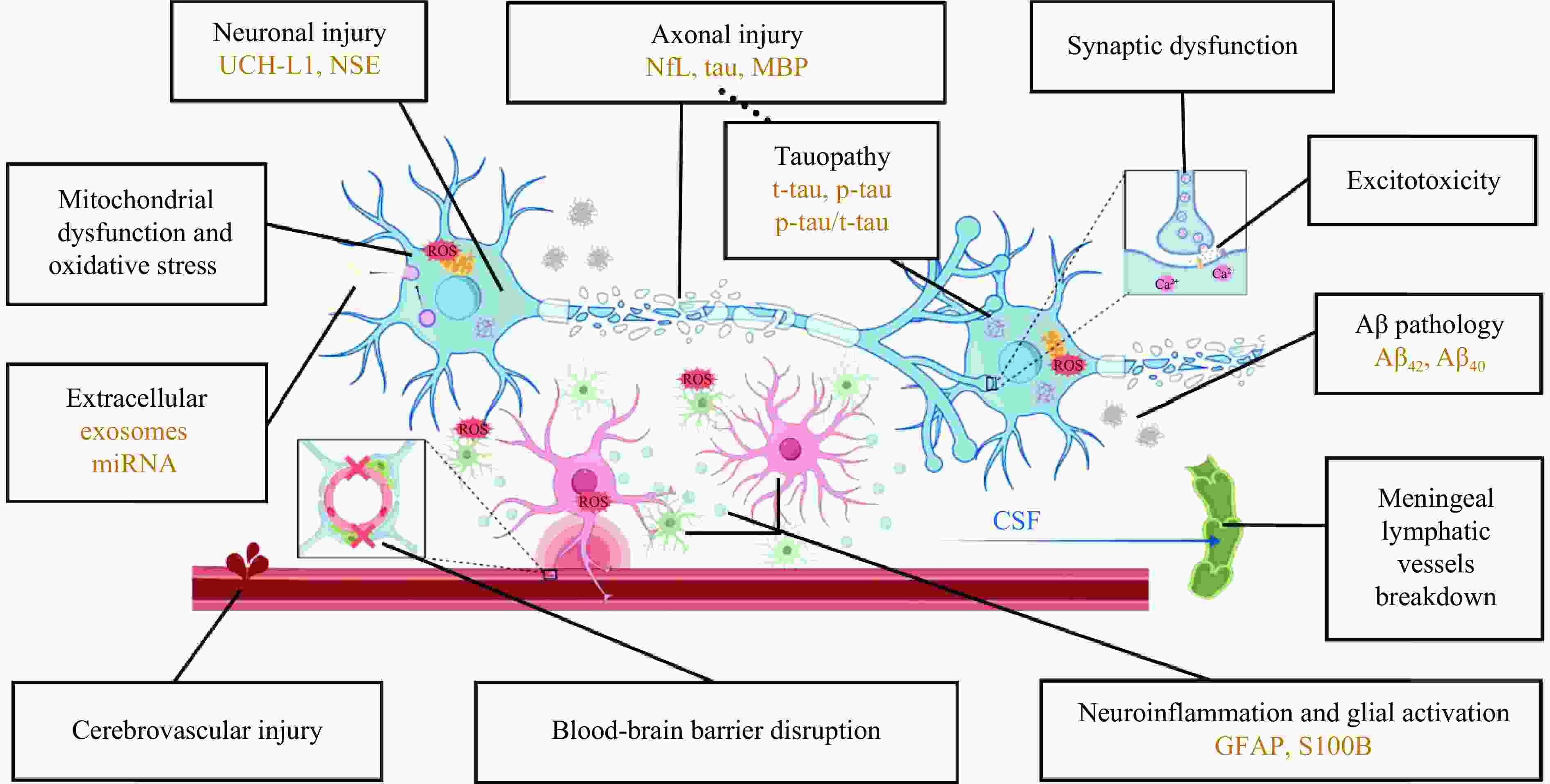
摘要: 爆炸冲击波性脑损伤(blast-induced traumatic brain injury,bTBI)是由爆炸时的冲击波对颅脑造成的损伤效应,伤者可表现出不同程度的躯体和行为障碍以及远期认知功能损害,是战时最常见的脑损伤类型。bTBI的发生机制复杂且尚未完全阐明。爆炸产生的冲击波作用于头部表面并在颅内传播,造成颅脑弥漫性损伤,从病理学层面可将bTBI分为原发性损伤和继发性损伤。冲击波的机械致伤效应会造成脑内结构的原发性受损,通常不可逆,只能采取有效的预防措施减少伤害。原发性损伤可引发一系列复杂的继发性级联反应,包括突触功能障碍、兴奋性毒性损伤、血脑屏障破坏、脑膜淋巴系统功能障碍、神经炎症、线粒体功能障碍、氧化应激反应、tau蛋白过度磷酸化和淀粉样蛋白-β病理改变等,可持续至伤后数天甚至慢性阶段,为临床治疗提供了干预的时间窗。轻度bTBI临床表现异质性高,影像学表现常呈阴性,早期诊断困难。但近年来bTBI的血液生物标志物取得长足进展,包括泛素C末端水解酶L1、神经元特异性烯醇化酶、神经丝蛋白轻链、磷酸化tau蛋白、髓鞘碱性蛋白、胶质纤维酸性蛋白、S100钙结合蛋白B和其他新兴生物标志物等,有望成为影像学阴性的bTBI的早期诊断和预后判断的潜在生物标志物。综上,本文重点综述了近年来关于bTBI的发生机制和生物标志物研究的前沿进展,并展望了未来的研究方向,以期为探索bTBI的发生机制、早期诊断策略和干预靶点提供新思路。Abstract: Blast-induced traumatic brain injury (bTBI) is defined as the damaging effect of the shock wave on the brain, which may cause behavioral impairment, physical symptoms and long-term cognitive impairment. Statistically, bTBI is the most common type of traumatic brain injury in combatants, but the mechanism has not been fully elucidated so far because of the high complexity of bTBI. When the shock wave produced during explosions acts on the surface of the skull and propagates within the head, it can lead to a diffuse damage to the brain. In terms of pathological mechanism, bTBI includes two aspects: primary injury and secondary injury. The mechanical injury effect of the shock wave generated by explosions can cause the primary injury of craniocerebral structures, which is usually irreversible and can be only prevented with effective measures. And the secondary injuries will be triggered by the primary injury after bTBI, which involve a series of complex cascades including synaptic dysfunction, excitotoxic injury, blood-brain barrier disruption, meningeal lymphatic system dysfunction, neuroinflammation, mitochondrial dysfunction, oxidative stress, tau protein hyperphosphorylation and amyloid-β pathological changes. And it can last for some time or even extend into the chronic stage after injury, providing a critical window for intervention. It is difficult to diagnose mild bTBI due to the high heterogeneity of clinical symptoms and the positive imaging manifestations. However, great progresses have been made in the research of blood biomarkers of bTBI in recent years, such as ubiquitin carboxyl-terminal hydrolase L1, neuron-specific enolase, neurofilament protein-light, hyperphosphorylated tau protein, myelin basic protein, glial fibrillary acidic protein, S100 calcium-binding protein B and other novel biomarkers. All of the above-mentioned biomarkers are expected to be effective means of early diagnosis and prognosis judgment of imaging-negative bTBI. In conclusion, this review focuses on the frontier progress of the pathogenesis and biomarkers of bTBI, and looks forward to future research directions in order to provide more new ideas for exploring the pathogenesis, early diagnosis strategies as well as intervention targets of bTBI.
-
Key words:
- blast-induced traumatic brain injury /
- shock wave /
- pathogenesis /
- biomarkers
-
表 1 bTBI严重程度分级
Table 1. Classification of bTBI severity
分级 格拉斯哥昏迷量表 意识丧失 意识改变 创伤后遗忘 神经影像学表现 轻度 13~15分 0~30 min ≤24 h 0~1 d 正常 中度 9~12分 30 min~24 h >24 h 1~7 d 正常或异常 重度 <9分 >24 h >24 h >7 d 正常或异常 -
[1] MORTIMER D S. Military traumatic brain injury [J]. Physical Medicine and Rehabilitation Clinics of North America, 2024, 35(3): 559–571. DOI: 10.1016/j.pmr.2024.02.008. [2] Office of the United Nations High Commissioner for Human Rights. Two-year update-protection of civilians: impact of hostilities on civilians since 24 February 2022 [EB/OL]. (2024-02-22)[2024-09-05]. https://www.ohchr.org/en/documents/country-reports/two-year-update-protection-civilians-impact-hostilities-civilians-24. [3] DENGLER B A, AGIMI Y, STOUT K, et al. Epidemiology, patterns of care and outcomes of traumatic brain injury in deployed military settings: implications for future military operations [J]. Journal of Trauma and Acute Care Surgery, 2022, 93(2): 220–228. DOI: 10.1097/TA.0000000000003497. [4] 夏照帆, 伍国胜. 创伤性脑损伤的临床研究进展 [J]. 第二军医大学学报, 2021, 42(2): 117–121. DOI: 10.16781/j.0258-879x.2021.02.0117.XIA Z F, WU G S. Traumatic brain injury: a clinical research progress [J]. Academic Journal of Second Military Medical University, 2021, 42(2): 117–121. DOI: 10.16781/j.0258-879x.2021.02.0117. [5] ROSENFELD J V, MCFARLANE A C, BRAGGE P, et al. Blast-related traumatic brain injury [J]. The Lancet Neurology, 2013, 12(9): 882–893. DOI: 10.1016/S1474-4422(13)70161-3. [6] 张文超, 王舒, 梁增友, 等. 爆炸冲击波致颅脑冲击伤数值模拟研究 [J]. 北京理工大学学报, 2022, 42(9): 881–890. DOI: 10.15918/j.tbit1001-0645.2021.191.ZHANG W C, WANG S, LIANG Z Y, et al. Numerical simulation on traumatic brain injury induced by blast waves [J]. Transactions of Beijing Institute of Technology, 2022, 42(9): 881–890. DOI: 10.15918/j.tbit1001-0645.2021.191. [7] HOGE C W, MCGURK D, THOMAS J L, et al. Mild traumatic brain injury in U. S. soldiers returning from Iraq [J]. New England Journal of Medicine, 2008, 358(5): 453–463. DOI: 10.1056/NEJMoa072972. [8] Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury [J]. Journal of Rehabilitation Research and Development, 2009, 46(6): CP1–68. [9] KIM S Y, YEH P H, OLLINGER J M, et al. Military-related mild traumatic brain injury: clinical characteristics, advanced neuroimaging, and molecular mechanisms [J]. Translational Psychiatry, 2023, 13(1): 289. DOI: 10.1038/s41398-023-02569-1. [10] MAAS A I R, MENON D K, ADELSON P D, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research [J]. The Lancet Neurology, 2017, 16(12): 987–1048. DOI: 10.1016/S1474-4422(17)30371-X. [11] LING G S, ECKLUND J M. Traumatic brain injury in modern war [J]. Current Opinion in Anesthesiology, 2011, 24(2): 124–130. DOI: 10.1097/ACO.0b013e32834458da. [12] CAPIZZI A, WOO J, VERDUZCO-GUTIERREZ M. Traumatic brain injury [J]. Medical Clinics of North America, 2020, 104(2): 213–238. DOI: 10.1016/j.mcna.2019.11.001. [13] DAVIS L E, PIRIO RICHARDSON S. Traumatic brain injury and subdural hematoma [M]//DAVIS L E, PIRIO RICHARDSON S. Fundamentals of Neurologic Disease. 2nd ed. New York: Springer, 2015: 225–233. DOI: 10.1007/978-1-4939-2359-5_18. [14] MAGNUSON J, LEONESSA F, LING G S F. Neuropathology of explosive blast traumatic brain injury [J]. Current Neurology and Neuroscience Reports, 2012, 12(5): 570–579. DOI: 10.1007/s11910-012-0303-6. [15] EME R. Neurobehavioral outcomes of mild traumatic brain injury: a mini review [J]. Brain Sciences, 2017, 7(5): 46. DOI: 10.3390/brainsci7050046. [16] CLAUSEN A N, BOUCHARD H C, Workgroup M A M, et al. Assessment of neuropsychological function in veterans with blast-related mild traumatic brain injury and subconcussive blast exposure [J]. Frontiers in Psychology, 2021, 12: 686330. DOI: 10.3389/fpsyg.2021.686330. [17] JURICK S M, CROCKER L D, MERRITT V C, et al. Independent and synergistic associations between TBI characteristics and PTSD symptom clusters on cognitive performance and postconcussive symptoms in Iraq and Afghanistan veterans [J]. The Journal of Neuropsychiatry and Clinical Neurosciences, 2021, 33(2): 98–108. DOI: 10.1176/appi.neuropsych.20050128. [18] LUCKE-WOLD B P, TURNER R C, LOGSDON A F, et al. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development [J]. Journal of Neurotrauma, 2014, 31(13): 1129–1138. DOI: 10.1089/neu.2013.3303. [19] GOLDSTEIN L E, FISHER A M, TAGGE C A, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model [J]. Science Translational Medicine, 2012, 4(134): 134ra60. DOI: 10.1126/scitranslmed.3003716. [20] PRIEMER D S, IACONO D, RHODES C H, et al. Chronic traumatic encephalopathy in the brains of military personnel [J]. New England Journal of Medicine, 2022, 386(23): 2169–2177. DOI: 10.1056/NEJMoa2203199. [21] HENION A K, WANG C P, AMUAN M, et al. Role of deployment history on the association between epilepsy and traumatic brain injury in post-9/11 era US veterans [J]. Neurology, 2023, 101(24): e2571–e2584. DOI: 10.1212/WNL.0000000000207943. [22] 康越, 马天, 黄献聪, 等. 颅脑爆炸伤数值模拟研究进展: 建模、力学机制及防护 [J]. 爆炸与冲击, 2023, 43(6): 061101. DOI: 10.11883/bzycj-2022-0521.KANG Y, MA T, HUANG X C, et al. Advances in numerical simulation of blast-induced traumatic brain injury: modeling, mechanical mechanism and protection [J]. Explosion and Shock Waves, 2023, 43(6): 061101. DOI: 10.11883/bzycj-2022-0521. [23] FIEVISOHN E, BAILEY Z, GUETTLER A, et al. Primary blast brain injury mechanisms: current knowledge, limitations, and future directions [J]. Journal of Biomechanical Engineering, 2018, 140(2): 020806. DOI: 10.1115/1.4038710. [24] DU Z B, LI Z J, WANG P, et al. Revealing the effect of skull deformation on intracranial pressure variation during the direct interaction between blast wave and surrogate head [J]. Annals of Biomedical Engineering, 2022, 50(9): 1038–1052. DOI: 10.1007/s10439-022-02982-5. [25] COURTNEY A C, COURTNEY M W. A thoracic mechanism of mild traumatic brain injury due to blast pressure waves [J]. Medical Hypotheses, 2009, 72(1): 76–83. DOI: 10.1016/j.mehy.2008.08.015. [26] 柳占立, 杜智博, 张家瑞, 等. 颅脑爆炸伤致伤机制及防护研究进展 [J]. 爆炸与冲击, 2022, 42(4): 041101. DOI: 10.11883/bzycj-2021-0053.LIU Z L, DU Z B, ZHANG J R, et al. Progress in the mechanism and protection of blast-induced traumatic brain injury [J]. Explosion and Shock Waves, 2022, 42(4): 041101. DOI: 10.11883/bzycj-2021-0053. [27] BAILEY Z S, HUBBARD W B, VANDEVORD P J. Cellular mechanisms and behavioral outcomes in blast-induced neurotrauma: comparing experimental setups [M]//KOBEISSY F H, DIXON C E, HAYES R L, et al. Injury Models of the Central Nervous System: Methods and Protocols. New York: Springer, 2016: 119–138. DOI: 10.1007/978-1-4939-3816-2_8. [28] 吴育寿, 柴家科. 脑冲击伤致伤机制和临床前治疗的研究进展 [J]. 中华创伤杂志, 2020, 36(5): 470–474. DOI: 10.3760/cma.j.issn.1001-8050.2020.05.014.WU Y S, CHAI J K. Research progress in mechanism and preclinical treatment for blast traumatic brain injury [J]. Chinese Journal of Trauma, 2020, 36(5): 470–474. DOI: 10.3760/cma.j.issn.1001-8050.2020.05.014. [29] ZIEBELL J M, MORGANTI-KOSSMANN M C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury [J]. Neurotherapeutics, 2010, 7(1): 22–30. DOI: 10.1016/j.nurt.2009.10.016. [30] PITT J, PITT Y, LOCKWICH J. Clinical and cellular aspects of traumatic brain injury [M]//GUPTA R C. Handbook of Toxicology of Chemical Warfare Agents. 3rd ed. Amsterdam: Elsevier, 2020: 745–765. DOI: 10.1016/B978-0-12-819090-6.00044-1. [31] NONAKA M, TAYLOR W W, BUKALO O, et al. Behavioral and myelin-related abnormalities after blast-induced mild traumatic brain injury in mice [J]. Journal of Neurotrauma, 2021, 38(11): 1551–1571. DOI: 10.1089/neu.2020.7254. [32] ARMSTRONG R C, SULLIVAN G M, PERL D P, et al. White matter damage and degeneration in traumatic brain injury [J]. Trends in Neurosciences, 2024, 47(9): 677–692. DOI: 10.1016/j.tins.2024.07.003. [33] GUEDES V A, KENNEY K, SHAHIM P, et al. Exosomal neurofilament light [J]. Neurology, 2020, 94(23): e2412–e2423. DOI: 10.1212/WNL.0000000000009577. [34] HILL C S, COLEMAN M P, MENON D K. Traumatic axonal injury: mechanisms and translational opportunities [J]. Trends in Neurosciences, 2016, 39(5): 311–324. DOI: 10.1016/j.tins.2016.03.002. [35] JAGODA A, PRABHU A, RIGGIO S. Behavioral and neurocognitive sequelae of concussion in the emergency department [M]//ZUN L S, NORDSTROM K, WILSON M P. Behavioral Emergencies for Healthcare Providers. Cham: Springer, 2021: 341–355. DOI: 10.1007/978-3-030-52520-0_35. [36] KRIEG J L, LEONARD A V, TURNER R J, et al. Identifying the phenotypes of diffuse axonal injury following traumatic brain injury [J]. Brain Sciences, 2023, 13(11): 1607. DOI: 10.3390/brainsci13111607. [37] JOHNSON V E, STEWART W, SMITH D H. Axonal pathology in traumatic brain injury [J]. Experimental Neurology, 2013, 246: 35–43. DOI: 10.1016/j.expneurol.2012.01.013. [38] FEHILY B, FITZGERALD M. Repeated mild traumatic brain injury: potential mechanisms of damage [J]. Cell Transplantation, 2017, 26(7): 1131–1155. DOI: 10.1177/0963689717714092. [39] POZO DEVOTO V M, LACOVICH V, FEOLE M, et al. Unraveling axonal mechanisms of traumatic brain injury [J]. Acta Neuropathologica Communications, 2022, 10(1): 140. DOI: 10.1186/s40478-022-01414-8. [40] CHEN Y, GU M, PATTERSON J, et al. Temporal alterations in cerebrovascular glycocalyx and cerebral blood flow after exposure to a high-intensity blast in rats [J]. International Journal of Molecular Sciences, 2024, 25(7): 3580. DOI: 10.3390/ijms25073580. [41] MCKEE A C, ROBINSON M E. Military-related traumatic brain injury and neurodegeneration [J]. Alzheimer’s & Dementia, 2014, 10(3S): S242–S253. DOI: 10.1016/j.jalz.2014.04.003. [42] TAGGE C A, FISHER A M, MINAEVA O V, et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model [J]. Brain, 2018, 141(2): 422–458. DOI: 10.1093/brain/awx350. [43] ELDER G A, GAMA SOSA M A, DE GASPERI R, et al. The neurovascular unit as a locus of injury in low-level blast-induced neurotrauma [J]. International Journal of Molecular Sciences, 2024, 25(2): 1150. DOI: 10.3390/ijms25021150. [44] ELDER G A, GAMA SOSA M A, DE GASPERI R, et al. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury [J]. Frontiers in Neurology, 2015, 6: 48. DOI: 10.3389/fneur.2015.00048. [45] GAMA SOSA M A, DE GASPERI R, PRYOR D, et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation [J]. Acta Neuropathologica Communications, 2021, 9(1): 167. DOI: 10.1186/s40478-021-01269-5. [46] MEABON J S, COOK D G, YAGI M, et al. Chronic elevation of plasma vascular endothelial growth factor-a (VEGF-A) is associated with a history of blast exposure [J]. Journal of the Neurological Sciences, 2020, 417: 117049. DOI: 10.1016/j.jns.2020.117049. [47] WANG Y, WEI Y L, REN M, et al. Blast exposure alters synaptic connectivity in the mouse auditory cortex [J]. Journal of Neurotrauma, 2024, 41(11/12): 1438–1449. DOI: 10.1089/neu.2023.0348. [48] RATLIFF W A, MERVIS R F, CITRON B A, et al. Effect of mild blast-induced TBI on dendritic architecture of the cortex and hippocampus in the mouse [J]. Scientific Reports, 2020, 10(1): 2206. DOI: 10.1038/s41598-020-59252-4. [49] KONAN L M, SONG H L, PENTECOST G, et al. Multi-focal neuronal ultrastructural abnormalities and synaptic alterations in mice after low-intensity blast exposure [J]. Journal of Neurotrauma, 2019, 36(13): 2117–2128. DOI: 10.1089/neu.2018.6260. [50] VOGEL Ⅲ E W, RWEMA S H, MEANEY D F, et al. Primary blast injury depressed hippocampal long-term potentiation through disruption of synaptic proteins [J]. Journal of Neurotrauma, 2017, 34(5): 1063–1073. DOI: 10.1089/neu.2016.4578. [51] VOGEL E W, EFFGEN G B, PATEL T P, et al. Isolated primary blast inhibits long-term potentiation in organotypic hippocampal slice cultures [J]. Journal of Neurotrauma, 2016, 33(7): 652–661. DOI: 10.1089/neu.2015.4045. [52] ALMEIDA M F, PIEHLER T, CARSTENS K E, et al. Distinct and dementia-related synaptopathy in the hippocampus after military blast exposures [J]. Brain Pathology, 2021, 31(3): e12936. DOI: 10.1111/bpa.12936. [53] JAMJOOM A A B, RHODES J, ANDREWS P J D, et al. The synapse in traumatic brain injury [J]. Brain, 2021, 144(1): 18–31. DOI: 10.1093/brain/awaa321. [54] KAPLAN G B, LEITE-MORRIS K A, WANG L, et al. Pathophysiological bases of comorbidity: traumatic brain injury and post-traumatic stress disorder [J]. Journal of Neurotrauma, 2018, 35(2): 210–225. DOI: 10.1089/neu.2016.4953. [55] CHEN S Y, SIEDHOFF H R, ZHANG H, et al. Low-intensity blast induces acute glutamatergic hyperexcitability in mouse hippocampus leading to long-term learning deficits and altered expression of proteins involved in synaptic plasticity and serine protease inhibitors [J]. Neurobiology of Disease, 2022, 165: 105634. DOI: 10.1016/j.nbd.2022.105634. [56] ORR T J, LESHA E, KRAMER A H, et al. Traumatic brain injury: a comprehensive review of biomechanics and molecular pathophysiology [J]. World Neurosurgery, 2024, 185: 74–88. DOI: 10.1016/j.wneu.2024.01.084. [57] BUTLER T R, SELF R L, SMITH K J, et al. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-d-asparate-type glutamate receptors [J]. Neuroscience, 2010, 165(2): 525–534. DOI: 10.1016/j.neuroscience.2009.10.018. [58] 刘子华, 胡博玄. 颅脑损伤后血脑屏障的损伤机制与检测方法新进展 [J]. 中国临床神经外科杂志, 2022, 27(3): 214–217. DOI: 10.13798/j.issn.1009-153X.2022.03.021. [59] BHOWMICK S, D’MELLO V, CARUSO D, et al. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury [J]. Experimental Neurology, 2019, 317: 260–270. DOI: 10.1016/j.expneurol.2019.03.014. [60] MICHINAGA S, KOYAMA Y. Pathophysiological responses and roles of astrocytes in traumatic brain injury [J]. International Journal of Molecular Sciences, 2021, 22(12): 6418. DOI: 10.3390/ijms22126418. [61] READNOWER R D, CHAVKO M, ADEEB S, et al. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury [J]. Journal of Neuroscience Research, 2010, 88(16): 3530–3539. DOI: 10.1002/jnr.22510. [62] DADGOSTAR E, RAHIMI S, NIKMANZAR S, et al. Aquaporin 4 in traumatic brain injury: from molecular pathways to therapeutic target [J]. Neurochemical Research, 2022, 47(4): 860–871. DOI: 10.1007/s11064-021-03512-w. [63] PUHAKKA N, DAS GUPTA S, LESKINEN S, et al. Proteomics of deep cervical lymph nodes after experimental traumatic brain injury [J]. Neurotrauma Reports, 2023, 4(1): 359–366. DOI: 10.1089/neur.2023.0008. [64] BOLTE A C, DUTTA A B, HURT M E, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis [J]. Nature Communications, 2020, 11(1): 4524. DOI: 10.1038/s41467-020-18113-4. [65] FREEDMAN M S, GNANAPAVAN S, BOOTH R A, et al. Guidance for use of neurofilament light chain as a cerebrospinal fluid and blood biomarker in multiple sclerosis management [J]. eBioMedicine, 2024, 101: 104970. DOI: 10.1016/j.ebiom.2024.104970. [66] LIAO J W, ZHANG M C, SHI Z C, et al. Improving the function of meningeal lymphatic vessels to promote brain edema absorption after traumatic brain injury [J]. Journal of Neurotrauma, 2023, 40(3/4): 383–394. DOI: 10.1089/neu.2022.0150. [67] BRAUN M, SEVAO M, KEIL S A, et al. Macroscopic changes in aquaporin-4 underlie blast traumatic brain injury-related impairment in glymphatic function [J]. Brain, 2024, 147(6): 2214–2229. DOI: 10.1093/brain/awae065. [68] TARASOFF-CONWAY J M, CARARE R O, OSORIO R S, et al. Clearance systems in the brain: implications for alzheimer disease [J]. Nature Reviews Neurology, 2015, 11(8): 457–470. DOI: 10.1038/nrneurol.2015.119. [69] LEVITES Y, DAMMER E B, RAN Y, et al. Integrative proteomics identifies a conserved Aβ amyloid responsome, novel plaque proteins, and pathology modifiers in Alzheimer’s disease [J]. Cell Reports Medicine, 2024, 5(8): 101669. DOI: 10.1016/j.xcrm.2024.101669. [70] SIMON D W, MCGEACHY M J, BAYIR H, et al. The far-reaching scope of neuroinflammation after traumatic brain injury [J]. Nature Reviews Neurology, 2017, 13(3): 171–191. DOI: 10.1038/nrneurol.2017.13. [71] MCKEE C A, LUKENS J R. Emerging roles for the immune system in traumatic brain injury [J]. Frontiers in Immunology, 2016, 7: 556. DOI: 10.3389/fimmu.2016.00556. [72] CORPS K N, ROTH T L, MCGAVERN D B. Inflammation and neuroprotection in traumatic brain injury [J]. JAMA Neurology, 2015, 72(3): 355–362. DOI: 10.1001/jamaneurol.2014.3558. [73] GUILHAUME-CORREA F, PICKRELL A M, VANDEVORD P J. The imbalance of astrocytic mitochondrial dynamics following blast-induced traumatic brain injury [J]. Biomedicines, 2023, 11(2): 329. DOI: 10.3390/biomedicines11020329. [74] SHIVELY S B, HORKAYNE-SZAKALY I, JONES R V, et al. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series [J]. The Lancet Neurology, 2016, 15(9): 944–953. DOI: 10.1016/S1474-4422(16)30057-6. [75] LIER J, ONDRUSCHKA B, BECHMANN I, et al. Fast microglial activation after severe traumatic brain injuries [J]. International Journal of Legal Medicine, 2020, 134(6): 2187–2193. DOI: 10.1007/s00414-020-02308-x. [76] RAMLACKHANSINGH A F, BROOKS D J, GREENWOOD R J, et al. Inflammation after trauma: microglial activation and traumatic brain injury [J]. Annals of Neurology, 2011, 70(3): 374–383. DOI: 10.1002/ana.22455. [77] JOHNSON V E, STEWART J E, BEGBIE F D, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury [J]. Brain, 2013, 136(1): 28–42. DOI: 10.1093/brain/aws322. [78] SONG H L, CHEN M, CHEN C, et al. Proteomic analysis and biochemical correlates of mitochondrial dysfunction after low-intensity primary blast exposure [J]. Journal of Neurotrauma, 2019, 36(10): 1591–1605. DOI: 10.1089/neu.2018.6114. [79] FRATI A, CERRETANI D, FIASCHI A I, et al. Diffuse axonal injury and oxidative stress: a comprehensive review [J]. International Journal of Molecular Sciences, 2017, 18(12): 2600. DOI: 10.3390/ijms18122600. [80] FESHARAKI-ZADEH A, DATTA D. An overview of preclinical models of traumatic brain injury (TBI): relevance to pathophysiological mechanisms [J]. Frontiers in Cellular Neuroscience, 2024, 18: 1371213. DOI: 10.3389/fncel.2024.1371213. [81] KURIAKOSE M, YOUNGER D, RAVULA A R, et al. Synergistic role of oxidative stress and blood-brain barrier permeability as injury mechanisms in the acute pathophysiology of blast-induced neurotrauma [J]. Scientific Reports, 2019, 9(1): 7717. DOI: 10.1038/s41598-019-44147-w. [82] FESHARAKI-ZADEH A. Oxidative stress in traumatic brain injury [J]. International Journal of Molecular Sciences, 2022, 23(21): 13000. DOI: 10.3390/ijms232113000. [83] ZHANG C N, LIU C, LI F J, et al. Extracellular mitochondria activate microglia and contribute to neuroinflammation in traumatic brain injury [J]. Neurotoxicity Research, 2022, 40(6): 2264–2277. DOI: 10.1007/s12640-022-00566-8. [84] ALONSO A D, COHEN L S, CORBO C, et al. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability [J]. Frontiers in Cellular Neuroscience, 2018, 12: 338. DOI: 10.3389/fncel.2018.00338. [85] CHEN M, SONG H L, CUI J K, et al. Proteomic profiling of mouse brains exposed to blast-induced mild traumatic brain injury reveals changes in axonal proteins and phosphorylated tau [J]. Journal of Alzheimer’s Disease, 2018, 66(2): 751–773. DOI: 10.3233/JAD-180726. [86] DICKSTEIN D L, DE GASPERI R, GAMA SOSA M A, et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure [J]. Molecular Psychiatry, 2021, 26(10): 5940–5954. DOI: 10.1038/s41380-020-0674-z. [87] BUTLER M L M D, DIXON E, STEIN T D, et al. Tau pathology in chronic traumatic encephalopathy is primarily neuronal [J]. Journal of Neuropathology & Experimental Neurology, 2022, 81(10): 773–780. DOI: 10.1093/jnen/nlac065. [88] KONDO A, SHAHPASAND K, MANNIX R, et al. Antibody against early driver of neurodegeneration cis p-tau blocks brain injury and tauopathy [J]. Nature, 2015, 523(7561): 431–436. DOI: 10.1038/nature14658. [89] FALCON B, ZIVANOV J, ZHANG W J, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules [J]. Nature, 2019, 568(7752): 420–423. DOI: 10.1038/s41586-019-1026-5. [90] BOUTTÉ A M, THANGAVELU B, NEMES J, et al. Neurotrauma biomarker levels and adverse symptoms among military and law enforcement personnel exposed to occupational overpressure without diagnosed traumatic brain injury [J]. JAMA Network Open, 2021, 4(4): e216445. DOI: 10.1001/jamanetworkopen.2021.6445. [91] LEIVA-SALINAS C, SINGH A, LAYFIELD E, et al. Early brain amyloid accumulation at PET in military instructors exposed to subconcussive blast injuries [J]. Radiology, 2023, 307(5): e221608. DOI: 10.1148/radiol.221608. [92] DEKOSKY S T, BLENNOW K, IKONOMOVIC M D, et al. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers [J]. Nature Reviews Neurology, 2013, 9(4): 192–200. DOI: 10.1038/nrneurol.2013.36. [93] MCKEE A C, STEIN T D, NOWINSKI C J, et al. The spectrum of disease in chronic traumatic encephalopathy [J]. Brain, 2013, 136(1): 43–64. DOI: 10.1093/brain/aws307. [94] PEREZ GARCIA G, DE GASPERI R, TSCHIFFELY A E, et al. Repetitive low-level blast exposure improves behavioral deficits and chronically lowers Aβ42 in an Alzheimer disease transgenic mouse model [J]. Journal of Neurotrauma, 2021, 38(22): 3146–3173. DOI: 10.1089/neu.2021.0184. [95] DE GASPERI R, GAMA SOSA M A, KIM S H, et al. Acute blast injury reduces brain abeta in two rodent species [J]. Frontiers in Neurology, 2012, 3: 177. DOI: 10.3389/fneur.2012.00177. [96] LI G, ILIFF J, SHOFER J, et al. CSF β-amyloid and tau biomarker changes in veterans with mild traumatic brain injury [J]. Neurology, 2024, 102(7): e209197. DOI: 10.1212/WNL.0000000000209197. [97] TURK K W, GEADA A, ALVAREZ V E, et al. A comparison between tau and amyloid-β cerebrospinal fluid biomarkers in chronic traumatic encephalopathy and Alzheimer disease [J]. Alzheimer’s Research & Therapy, 2022, 14(1): 28. DOI: 10.1186/s13195-022-00976-y. [98] SUGARMAN M A, MCKEE A C, STEIN T D, et al. Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia [J]. Alzheimer’s & Dementia, 2019, 15(5): 686–698. DOI: 10.1016/j.jalz.2018.12.015. [99] WEINER M W, HARVEY D, LANDAU S M, et al. Traumatic brain injury and post-traumatic stress disorder are not associated with Alzheimer’s disease pathology measured with biomarkers [J]. Alzheimer’s & Dementia, 2023, 19(3): 884–895. DOI: 10.1002/alz.12712. [100] ASHWORTH E R, BAXTER D, GIBB I E. Blast traumatic brain injury [M]//BULL A M J, CLASPER J, MAHONEY P F. Blast Injury Science and Engineering. Cham: Springer, 2022: 231-236. DOI: 10.1007/978-3-031-10355-1_22. [101] GAVETT B E, CANTU R C, SHENTON M, et al. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions [J]. Current Opinion in Neurology, 2011, 24(6): 525–531. DOI: 10.1097/WCO.0b013e32834cd477. [102] RANZENBERGER L R, DAS J M, SNYDER T. Diffusion tensor imaging [M/OL]//StatPearls. Treasure Island (FL): StatPearls Publishing, 2024[2024-05-21]. http://www.ncbi.nlm.nih.gov/books/NBK537361/. [103] GRANT M, LIU J J, WINTERMARK M, et al. Current state of diffusion-weighted imaging and diffusion tensor imaging for traumatic brain injury prognostication [J]. Neuroimaging Clinics of North America, 2023, 33(2): 279–297. DOI: 10.1016/j.nic.2023.01.004. [104] JORGE R E, ACION L, WHITE T, et al. White matter abnormalities in veterans with mild traumatic brain injury [J]. American Journal of Psychiatry, 2012, 169(12): 1284–1291. DOI: 10.1176/appi.ajp.2012.12050600. [105] STONE J R, AVANTS B B, TUSTISON N J, et al. Functional and structural neuroimaging correlates of repetitive low-level blast exposure in career breachers [J]. Journal of Neurotrauma, 2020, 37(23): 2468–2481. DOI: 10.1089/neu.2020.7141. [106] PINTO M S, WINZECK S, KORNAROPOULOS E N, et al. Use of support vector machines approach via combat harmonized diffusion tensor imaging for the diagnosis and prognosis of mild traumatic brain injury: a CENTER-TBI study [J]. Journal of Neurotrauma, 2023, 40(13/14): 1317–1338. DOI: 10.1089/neu.2022.0365. [107] GRAHAM N S N, JOLLY A, ZIMMERMAN K, et al. Diffuse axonal injury predicts neurodegeneration after moderate–severe traumatic brain injury [J]. Brain, 2020, 143(12): 3685–3698. DOI: 10.1093/brain/awaa316. [108] AGOSTON D V, KAMNAKSH A. Modeling the neurobehavioral consequences of blast-induced traumatic brain injury spectrum disorder and identifying related biomarkers [M]//KOBEISSY F H. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton: CRC Press, 2015. [109] ZETTERBERG H, BLENNOW K. Fluid biomarkers for mild traumatic brain injury and related conditions [J]. Nature Reviews Neurology, 2016, 12(10): 563–574. DOI: 10.1038/nrneurol.2016.127. [110] ZETTERBERG H, SMITH D H, BLENNOW K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood [J]. Nature Reviews Neurology, 2013, 9(4): 201–210. DOI: 10.1038/nrneurol.2013.9. [111] GHAITH H S, NAWAR A A, GABRA M D, et al. A literature review of traumatic brain injury biomarkers [J]. Molecular Neurobiology, 2022, 59(7): 4141–4158. DOI: 10.1007/s12035-022-02822-6. [112] SVETLOV S I, LARNER S F, KIRK D R, et al. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury [J]. Journal of Neurotrauma, 2009, 26(6): 913–921. DOI: 10.1089/neu.2008.0609. [113] AGOSTON D V, ELSAYED M. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder [J]. Frontiers in Neurology, 2012, 3: 107. DOI: 10.3389/fneur.2012.00107. [114] BAZARIAN J J, BIBERTHALER P, WELCH R D, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study [J]. The Lancet Neurology, 2018, 17(9): 782–789. DOI: 10.1016/S1474-4422(18)30231-X. [115] TRIVEDI D, FORSSTEN M P, CAO Y, et al. Screening performance of S100 calcium-binding protein B, glial fibrillary acidic protein, and ubiquitin C-terminal hydrolase L1 for intracranial injury within six hours of injury and beyond [J]. Journal of Neurotrauma, 2024, 41(3/4): 349–358. DOI: 10.1089/neu.2023.0322. [116] CARR W, YARNELL A M, ONG R, et al. Ubiquitin carboxy-terminal hydrolase-L1 as a serum neurotrauma biomarker for exposure to occupational low-level blast [J]. Frontiers in Neurology, 2015, 6: 49. DOI: 10.3389/fneur.2015.00049. [117] BOUTTÉ A M, THANGAVELU B, LAVALLE C R, et al. Brain-related proteins as serum biomarkers of acute, subconcussive blast overpressure exposure: a cohort study of military personnel [J]. PLoS One, 2019, 14(8): e0221036. DOI: 10.1371/journal.pone.0221036. [118] KOCIK V I, DENGLER B A, RIZZO J A, et al. A narrative review of existing and developing biomarkers in acute traumatic brain injury for potential military deployed use [J]. Military Medicine, 2024, 189(5/6): e1374–e1380. DOI: 10.1093/milmed/usad433. [119] KORLEY F K, JAIN S, SUN X Y, et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: an observational cohort study [J]. The Lancet Neurology, 2022, 21(9): 803–813. DOI: 10.1016/S1474-4422(22)00256-3. [120] HELMRICH I R A R, CZEITER E, AMREIN K, et al. Incremental prognostic value of acute serum biomarkers for functional outcome after traumatic brain injury (CENTER-TBI): an observational cohort study [J]. The Lancet Neurology, 2022, 21(9): 792–802. DOI: 10.1016/S1474-4422(22)00218-6. [121] PUCCIO A M, YUE J K, KORLEY F K, et al. Diagnostic utility of glial fibrillary acidic protein beyond 12 hours after traumatic brain injury: a TRACK-TBI study [J]. Journal of Neurotrauma, 2024, 41(11/12): 1353–1363. DOI: 10.1089/neu.2023.0186. [122] BÖHMER A E, OSES J P, SCHMIDT A P, et al. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury [J]. Neurosurgery, 2011, 68(6): 1624–1631. DOI: 10.1227/NEU.0b013e318214a81f. [123] POWELL J R, BOLTZ A J, DECICCO J P, et al. Neuroinflammatory biomarkers associated with mild traumatic brain injury history in special operations forces combat soldiers [J]. Journal of Head Trauma Rehabilitation, 2020, 35(5): 300–307. DOI: 10.1097/HTR.0000000000000598. [124] MERCIER E, TARDIF P A, CAMERON P A, et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review [J]. Brain Injury, 2018, 32(1): 29–40. DOI: 10.1080/02699052.2017.1385097. [125] MERCIER E, BOUTIN A, SHEMILT M, et al. Predictive value of neuron-specific enolase for prognosis in patients with moderate or severe traumatic brain injury: a systematic review and meta-analysis [J]. Canadian Medical Association Open Access Journal, 2016, 4(3): E371–E382. DOI: 10.9778/cmajo.20150061. [126] RICHTER S, WINZECK S, CZEITER E, et al. Serum biomarkers identify critically ill traumatic brain injury patients for MRI [J]. Critical Care, 2022, 26(1): 369. DOI: 10.1186/s13054-022-04250-3. [127] CLARKE G J B, FOLLESTAD T, SKANDSEN T, et al. Chronic immunosuppression across 12 months and high ability of acute and subacute CNS-injury biomarker concentrations to identify individuals with complicated mTBI on acute CT and MRI [J]. Journal of Neuroinflammation, 2024, 21(1): 109. DOI: 10.1186/s12974-024-03094-8. [128] SHAHIM P, POLITIS A, VAN DER MERWE A, et al. Neurofilament light as a biomarker in traumatic brain injury [J]. Neurology, 2020, 95(6): e610–e622. DOI: 10.1212/WNL.0000000000009983. [129] VORN R, NAUNHEIM R, LAI C, et al. Elevated axonal protein markers following repetitive blast exposure in military personnel [J]. Frontiers in Neuroscience, 2022, 16: 853616. DOI: 10.3389/fnins.2022.853616. [130] GRAHAM N S N, ZIMMERMAN K A, MORO F, et al. Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury [J]. Science Translational Medicine, 2021, 13(613): eabg9922. DOI: 10.1126/scitranslmed.abg9922. [131] SHAHIM P, PHAM D L, VAN DER MERWE A J, et al. Serum NFL and GFAP as biomarkers of progressive neurodegeneration in TBI [J]. Alzheimer’s & Dementia, 2024, 20(7): 4663–4676. DOI: 10.1002/alz.13898. [132] HALICKI M J, HIND K, CHAZOT P L. Blood-based biomarkers in the diagnosis of chronic traumatic encephalopathy: research to date and future directions [J]. International Journal of Molecular Sciences, 2023, 24(16): 12556. DOI: 10.3390/ijms241612556. [133] RUBENSTEIN R, CHANG B G, YUE J K, et al. Comparing plasma phospho tau, total tau, and phospho tau-total tau ratio as acute and chronic traumatic brain injury biomarkers [J]. JAMA Neurology, 2017, 74(9): 1063–1072. DOI: 10.1001/jamaneurol.2017.0655. [134] LANGE R T, LIPPA S, BRICKELL T A, et al. Serum tau, neurofilament light chain, glial fibrillary acidic protein, and ubiquitin carboxyl-terminal hydrolase L1 are associated with the chronic deterioration of neurobehavioral symptoms after traumatic brain injury [J]. Journal of Neurotrauma, 2023, 40(5/6): 482–492. DOI: 10.1089/neu.2022.0249. [135] SHI H, HU X M, LEAK R K, et al. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury [J]. Experimental Neurology, 2015, 272: 17–25. DOI: 10.1016/j.expneurol.2015.03.017. [136] MEHTA T, FAYYAZ M, GILER G E, et al. Current trends in biomarkers for traumatic brain injury [J]. Open Access Journal of Neurology & Neurosurgery, 2020, 12(4): 86–94. [137] KIM H J, TSAO J W, STANFILL A G. The current state of biomarkers of mild traumatic brain injury [J]. JCI Insight, 2018, 3(1): e97105. DOI: 10.1172/jci.insight.97105. [138] JETER C B, HERGENROEDER G W, HYLIN M J, et al. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion [J]. Journal of Neurotrauma, 2013, 30(8): 657–670. DOI: 10.1089/neu.2012.2439. [139] SHANG Y J, WANG Y X, GUO Y D, et al. Analysis of the risk of traumatic brain injury and evaluation neurogranin and myelin basic protein as potential biomarkers of traumatic brain injury in postmortem examination [J]. Forensic Science, Medicine and Pathology, 2022, 18(3): 288–298. DOI: 10.1007/s12024-022-00459-4. [140] BOHNERT S, WIRTH C, SCHMITZ W, et al. Myelin basic protein and neurofilament h in postmortem cerebrospinal fluid as surrogate markers of fatal traumatic brain injury [J]. International Journal of Legal Medicine, 2021, 135(4): 1525–1535. DOI: 10.1007/s00414-021-02606-y. [141] LIU Z T, LIU C W, MA K G. Retrospective study on the correlation between serum MIF level and the condition and prognosis of patients with traumatic head injury [J]. PeerJ, 2023, 11: e15933. DOI: 10.7717/peerj.15933. [142] ABDELHAK A, FOSCHI M, ABU-RUMEILEH S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders [J]. Nature Reviews Neurology, 2022, 18(3): 158–172. DOI: 10.1038/s41582-021-00616-3. [143] MENDITTO V G, MORETTI M, BABINI L, et al. Minor head injury in anticoagulated patients: performance of biomarkers S100B, NSE, GFAP, UCH-L1 and alinity TBI in the detection of intracranial injury. a prospective observational study [J]. Clinical Chemistry and Laboratory Medicine, 2024, 62(7): 1376–1382. DOI: 10.1515/cclm-2023-1169. [144] GIL-JARDINÉ C, PAYEN J F, BERNARD R, et al. Management of patients suffering from mild traumatic brain injury 2023 [J]. Anaesthesia Critical Care & Pain Medicine, 2023, 42(4): 101260. DOI: 10.1016/j.accpm.2023.101260. [145] TSCHIFFELY A E, STATZ J K, EDWARDS K A, et al. Assessing a blast-related biomarker in an operational community: glial fibrillary acidic protein in experienced breachers [J]. Journal of Neurotrauma, 2020, 37(8): 1091–1096. DOI: 10.1089/neu.2019.6512. [146] THANGAVELU B, LAVALLE C R, EGNOTO M J, et al. Overpressure exposure from 50-caliber rifle training is associated with increased amyloid beta peptides in serum [J]. Frontiers in Neurology, 2020, 11: 620. DOI: 10.3389/fneur.2020.00620. [147] PIERCE M E, HAYES J, HUBER B R, et al. Plasma biomarkers associated with deployment trauma and its consequences in post-9/11 era veterans: initial findings from the TRACTS longitudinal cohort [J]. Translational Psychiatry, 2022, 12(1): 80. DOI: 10.1038/s41398-022-01853-w. [148] TOMPKINS P, TESIRAM Y, LERNER M, et al. Brain injury: neuro-inflammation, cognitive deficit, and magnetic resonance imaging in a model of blast induced traumatic brain injury [J]. Journal of Neurotrauma, 2013, 30(22): 1888–1897. DOI: 10.1089/neu.2012.2674. [149] KAWAUCHI S, KONO A, MURAMATSU Y, et al. Meningeal damage and interface astroglial scarring in the rat brain exposed to a laser-induced shock wave(s) [J]. Journal of Neurotrauma, 2024, 41(15/16): e2039–e2053. DOI: 10.1089/neu.2023.0572. [150] SAJJA V S S S, HUBBARD W B, HALL C S, et al. Enduring deficits in memory and neuronal pathology after blast-induced traumatic brain injury [J]. Scientific Reports, 2015, 5(1): 15075. DOI: 10.1038/srep15075. [151] UNDÉN J, INGEBRIGTSEN T, ROMNER B, et al. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update [J]. BMC Medicine, 2013, 11: 50. DOI: 10.1186/1741-7015-11-50. [152] ORIS C, KAHOUADJI S, BOUVIER D, et al. Blood biomarkers for the management of mild traumatic brain injury in clinical practice [J]. Clinical Chemistry, 2024, 70(8): 1023–1036. DOI: 10.1093/clinchem/hvae049. [153] UNDÉN J, ROMNER B. A new objective method for CT triage after minor head injury-serum S100B [J]. Scandinavian Journal of Clinical and Laboratory Investigation, 2009, 69(1): 13–17. DOI: 10.1080/00365510802651833. [154] 刘宇晨. 细胞外囊泡的生物学功能及其作为体内小核糖核酸药物递送载体的研究 [D]. 南京: 南京大学, 2016: 10–16.LIU Y C. The biological functions of extracellular vesicle and its utilization as small RNA carrier in vivo [D]. Nanjing: Nanjing University, 2016: 10–16. [155] FLYNN S, LEETE J, SHAHIM P, et al. Extracellular vesicle concentrations of glial fibrillary acidic protein and neurofilament light measured 1 year after traumatic brain injury [J]. Scientific Reports, 2021, 11(1): 3896. DOI: 10.1038/s41598-021-82875-0. [156] SCHINDLER C R, HÖRAUF J A, WEBER B, et al. Identification of novel blood-based extracellular vesicles biomarker candidates with potential specificity for traumatic brain injury in polytrauma patients [J]. Frontiers in Immunology, 2024, 15: 1347767. DOI: 10.3389/fimmu.2024.1347767. [157] XU X J, GE Q Q, YANG M S, et al. Neutrophil-derived interleukin-17A participates in neuroinflammation induced by traumatic brain injury [J]. Neural Regeneration Research, 2023, 18(5): 1046–1051. DOI: 10.4103/1673-5374.355767. [158] HUANG X T, XU X J, WANG C, et al. Using bioinformatics technology to mine the expression of serum exosomal miRNA in patients with traumatic brain injury [J]. Frontiers in Neuroscience, 2023, 17: 1145307. DOI: 10.3389/fnins.2023.1145307. [159] REDELL J B, MOORE A N, WARD N H, et al. Human traumatic brain injury alters plasma microrna levels [J]. Journal of Neurotrauma, 2010, 27(12): 2147–2156. DOI: 10.1089/neu.2010.1481. [160] DI PIETRO V, RAGUSA M, DAVIES D, et al. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury [J]. Journal of Neurotrauma, 2017, 34(11): 1948–1956. DOI: 10.1089/neu.2016.4857. [161] YANG Y J, WANG Y, LI P P, et al. Serum exosomes miR-206 and miR-549a-3p as potential biomarkers of traumatic brain injury [J]. Scientific Reports, 2024, 14(1): 10082. DOI: 10.1038/s41598-024-60827-8. [162] JOHNSON J J, LOEFFERT A C, STOKES J, et al. Association of salivary microrna changes with prolonged concussion symptoms [J]. JAMA Pediatrics, 2018, 172(1): 65–73. DOI: 10.1001/jamapediatrics.2017.3884. [163] BHOMIA M, BALAKATHIRESAN N S, WANG K K, et al. A panel of serum miRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans [J]. Scientific Reports, 2016, 6(1): 28148. DOI: 10.1038/srep28148. [164] TAHERI S, TANRIVERDI F, ZARARSIZ G, et al. Circulating microRNAs as potential biomarkers for traumatic brain injury-induced hypopituitarism [J]. Journal of Neurotrauma, 2016, 33(20): 1818–1825. DOI: 10.1089/neu.2015.4281. -






 下载:
下载: