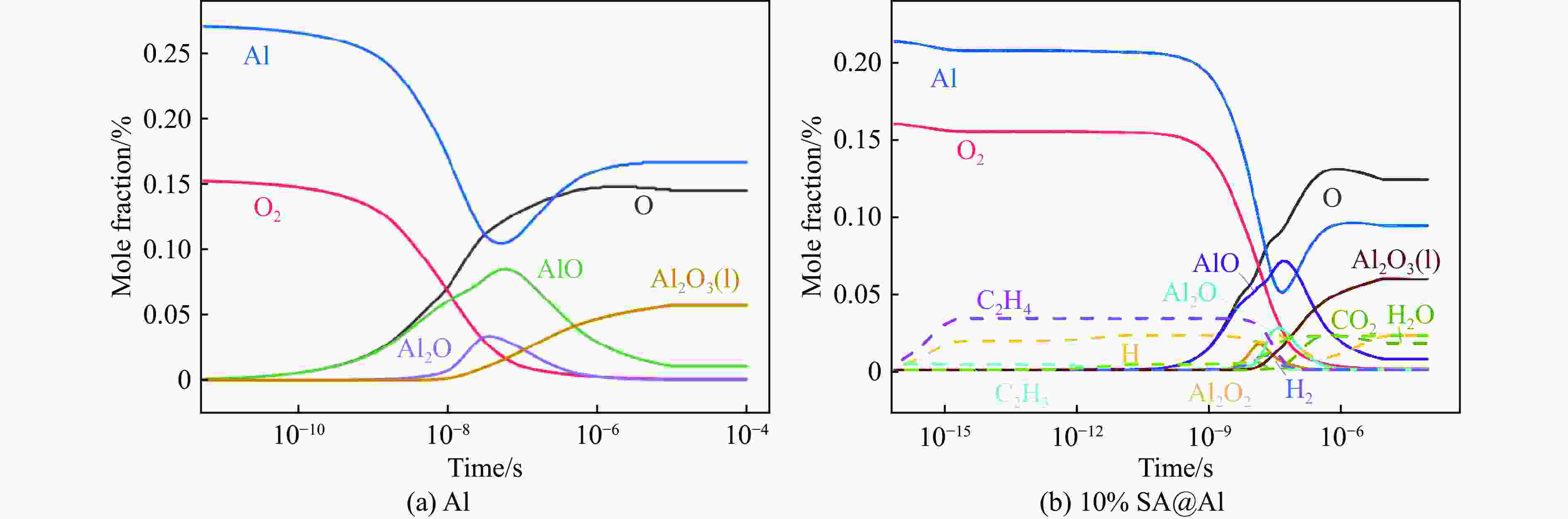
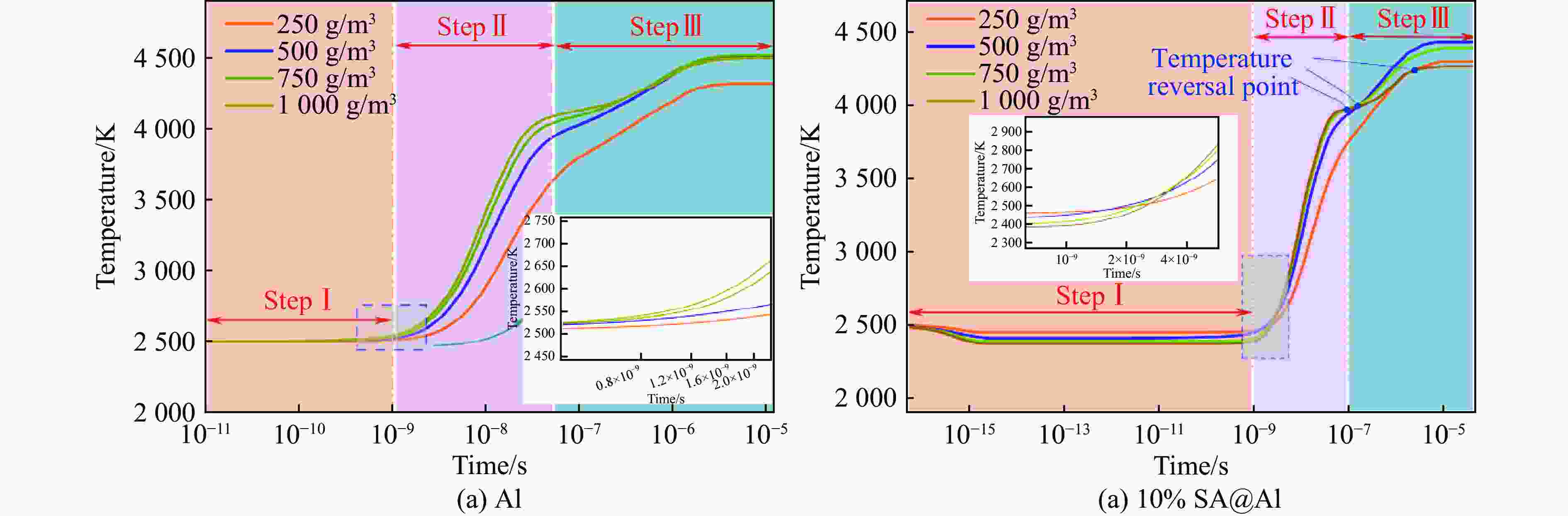
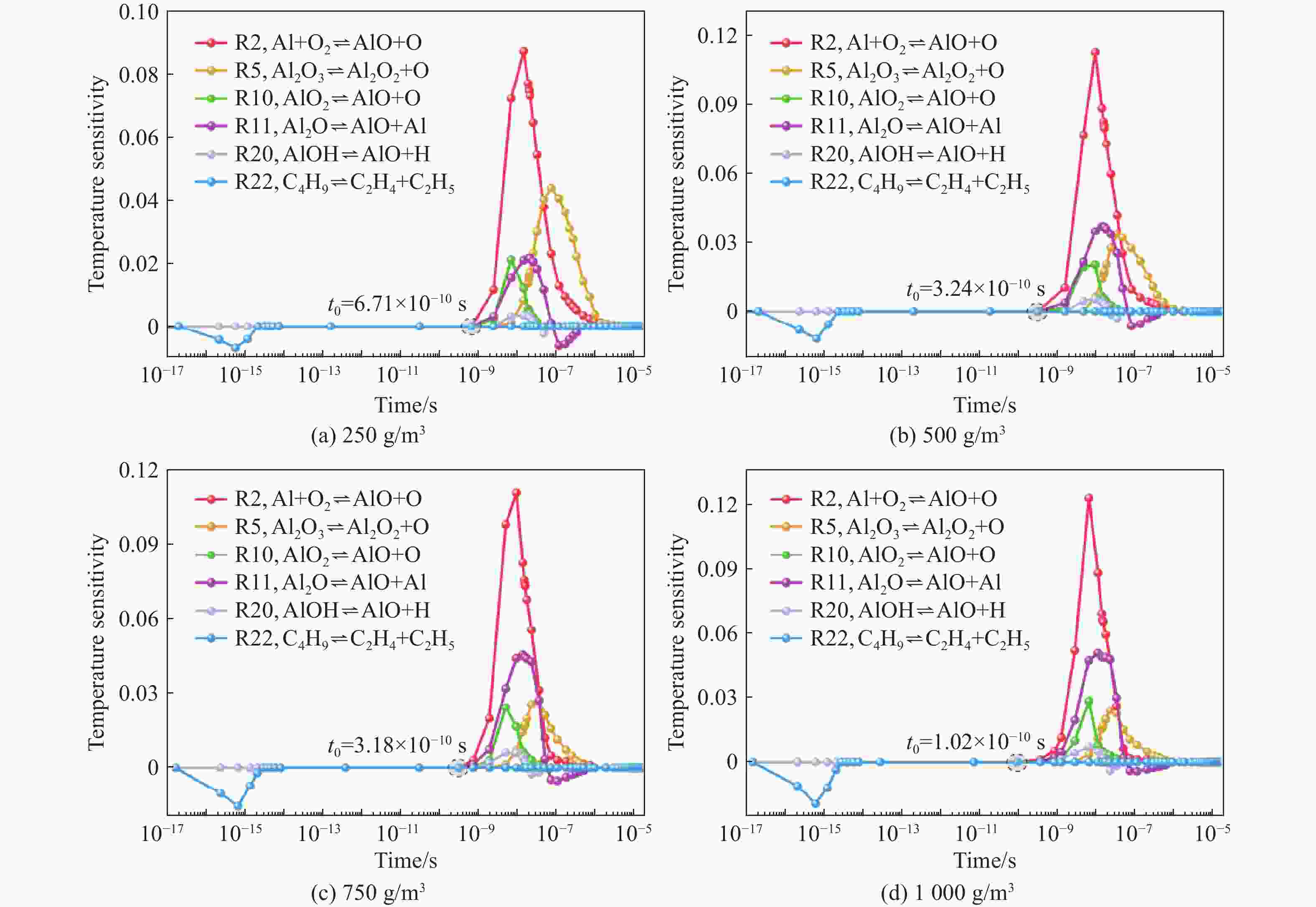
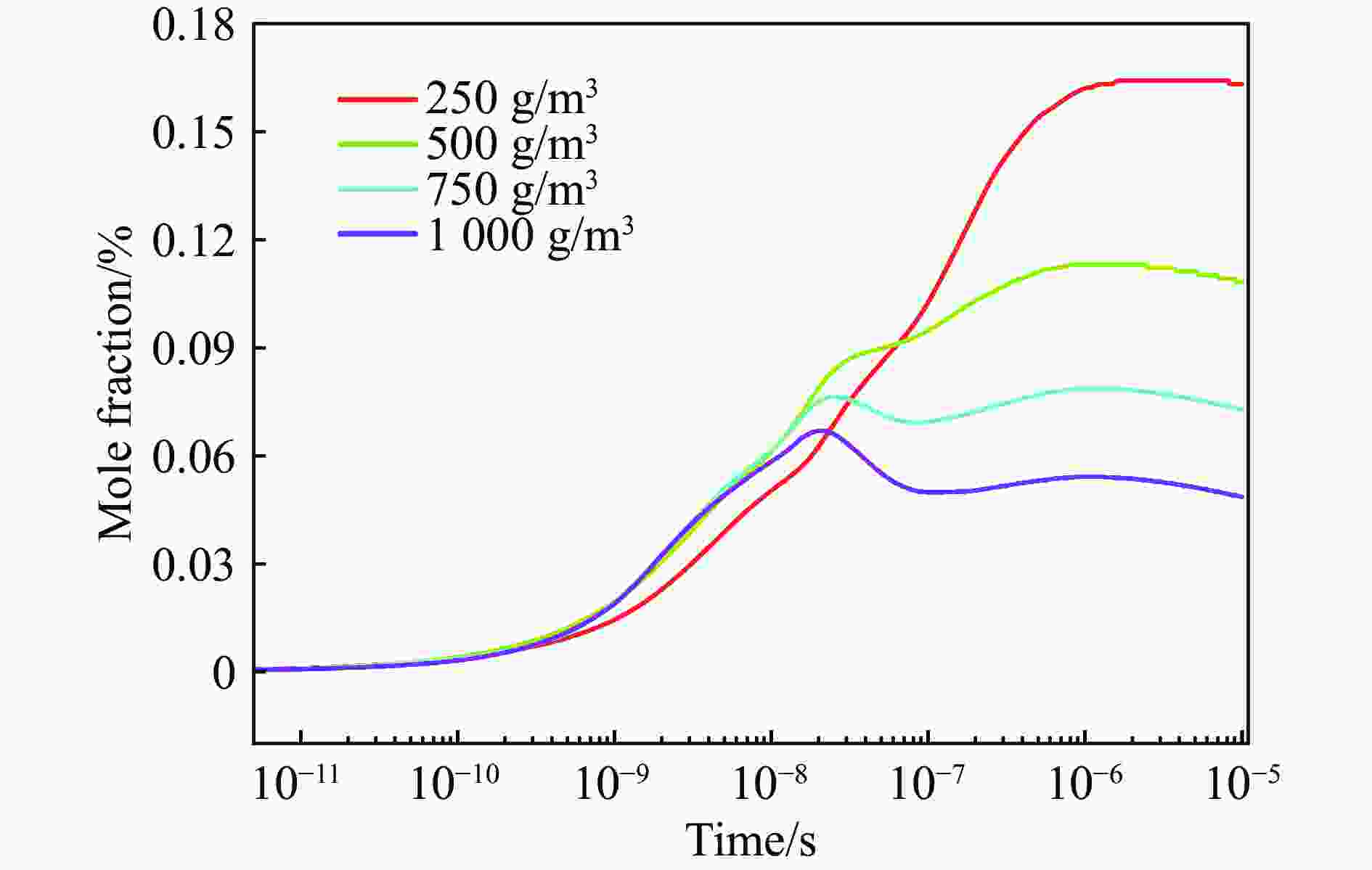
| [1] |
LIANG L, GUO X D, LIAO X, et al. Improve the interfacial adhesion, corrosion resistance and combustion properties of aluminum powder by modification of nickel and dopamine [J]. Applied Surface Science, 2020, 508: 144790. DOI: 10.1016/j.apsusc.2019.144790.
|
| [2] |
尚方静, 王文先, 杨涛, 等. 镍包覆氧化铝增强铁基复合材料的界面行为及其耐磨性 [J]. 稀有金属材料与工程, 2022, 51(2): 422–428. DOI: 10.12442/j.issn.1002-185X.20200982.SHANG F J, WANG W X, YANG T, et al. Interaction mechanism and wear resistance of Ni-encapsulated Al2O3 particles reinforced iron matrix composites [J]. Rare Metal Materials and Engineering, 2022, 51(2): 422–428. DOI: 10.12442/j.issn.1002-185X.20200982.
|
| [3] |
SUN J, PANTOYA M L, SIMON S L. Dependence of size and size distribution on reactivity of aluminum nanoparticles in reactions with oxygen and MoO3 [J]. Thermochimica Acta, 2006, 444(2): 117–127. DOI: 10.1016/j.tca.2006.03.001.
|
| [4] |
GROMOV A A, FÖRTER-BARTH U, TEIPEL U. Aluminum nanopowders produced by electrical explosion of wires and passivated by non-inert coatings: characterisation and reactivity with air and water [J]. Powder Technology, 2006, 164(2): 111–115. DOI: 10.1016/j.powtec.2006.03.003.
|
| [5] |
LIU H, YE H Q, ZHANG Y C. Preparation and characterization of PMMA/flaky aluminum composite particle in the presence of MPS [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 315(1/2/3): 1–6. DOI: 10.1016/j.colsurfa.2007.06.057.
|
| [6] |
CHEN J S, CHEN K, SHI W X, et al. The preparation of novel core-shell suppressor and its suppression mechanism on coal dust explosion flame [J]. Fuel, 2022, 313: 122997. DOI: 10.1016/j.fuel.2021.122997.
|
| [7] |
ESCOT BOCANEGRA P, CHAUVEAU C, GÖKALP I. Experimental studies on the burning of coated and uncoated micro and nano-sized aluminium particles [J]. Aerospace Science and Technology, 2007, 11(1): 33–38. DOI: 10.1016/j.ast.2006.10.005.
|
| [8] |
JIANG H P, BI M S, ZHANG J K, et al. Explosion hazard and prevention of Al-Ni mechanical alloy powders [J]. Journal of Loss Prevention in the Process Industries, 2022, 75: 104714. DOI: 10.1016/j.jlp.2021.104714.
|
| [9] |
LYU X, GAO Y, CUI Y S, et al. Study of ignition and combustion characteristics of kerosene-based nanofluid fuel containing n-Al/CuO thermite [J]. Fuel, 2023, 331: 125778. DOI: 10.1016/j.fuel.2022.125778.
|
| [10] |
XIAO F, LIU Z H, LIANG T X, et al. Establishing the interface layer on the aluminum surface through the self-assembly of tannic acid (TA): improving the ignition and combustion properties of aluminum [J]. Chemical Engineering Journal, 2021, 420(Pt3): 130523. DOI: 10.1016/j.cej.2021.130523.
|
| [11] |
XU J T, HUANG L, JIANG H P, et al. Explosion characteristics of aluminum-based activated fuels containing fluorine [J]. Defence Technology, 2023, 20: 34–43. DOI: 10.1016/j.dt.2021.12.008.
|
| [12] |
KWON Y S, GROMOV A A, STROKOVA J I. Passivation of the surface of aluminum nanopowders by protective coatings of the different chemical origin [J]. Applied Surface Science, 2007, 253(12): 5558–5564. DOI: 10.1016/j.apsusc.2006.12.124.
|
| [13] |
商琦伟. 铝粉/氟化物的研制及其性能研究 [D]. 太原: 中北大学, 2023. DOI: 10.27470/d.cnki.ghbgc.2023.001368.SHANG Q W. Preparation and properties of aluminum powder/fluoride [D]. Taiyuan: North University of China, 2023. DOI: 10.27470/d.cnki.ghbgc.2023.001368.
|
| [14] |
LI N, ZHANG Y S, GUO R, et al. Effect of stearic acid coating on the explosion characteristics of aluminum dust [J]. Fuel, 2022, 320: 123880. DOI: 10.1016/j.fuel.2022.123880.
|
| [15] |
SOSSI A, DURANTI E, PARAVAN C, et al. Non-isothermal oxidation of aluminum nanopowder coated by hydrocarbons and fluorohydrocarbons [J]. Applied Surface Science, 2013, 271: 337–343. DOI: 10.1016/j.apsusc.2013.01.197.
|
| [16] |
堵同宽, 朱宝忠, 李浩, 等. 硬脂酸包覆纳米铝粉燃烧特性 [J]. 安徽工业大学学报(自然科学版), 2016, 33(1): 23–27. DOI: 10.3969/j.issn.1671-7872.2016.01.006.DU T K, ZHU B Z, LI H, et al. Combustion characteristics of stearic acid-coated aluminum nanopowder [J]. Journal of Anhui University of Technology (Natural Science), 2016, 33(1): 23–27. DOI: 10.3969/j.issn.1671-7872.2016.01.006.
|
| [17] |
GUO R, LI N, ZHANG X Y, ZHANG Y S, et al. Suppression mechanism of micron/nano PMMA dust flame based on thermal analysis [J]. Advanced Powder Technology, 2022, 33(12): 103848. DOI: 10.1016/j.apt.2022.103848.
|
| [18] |
张延松, 李南, 郭瑞, 等. 月桂酸与硬脂酸粉尘爆炸过程热解动力学与火焰传播特性关系 [J]. 爆炸与冲击, 2022, 42(7): 075402. DOI: 10.11883/bzycj-2021-0470.ZHANG Y S, LI N, GUO R, et al. Relationship between pyrolysis kinetics and flame propagation characteristics of lauric acid and stearic acid dust [J]. Explosion and Shock Waves, 2022, 42(7): 075402. DOI: 10.11883/bzycj-2021-0470.
|
| [19] |
KIM K T, KIM D W, KIM S H, et al. Synthesis and improved explosion behaviors of aluminum powders coated with nano-sized nickel film [J]. Applied Surface Science, 2017, 415: 104–108. DOI: 10.1016/j.apsusc.2016.11.056.
|
| [20] |
ZHU C C, GAO W, JIANG H P, et al. A comparative investigation of the explosion mechanism of metal hydride AlH3 dust and Al/H2 mixture [J]. International Journal of Hydrogen Energy, 2024, 50: 1296–1305. DOI: 10.1016/j.ijhydene.2023.10.178.
|
| [21] |
LI Q Z, WANG K, ZHENG Y N, et al. Explosion severity of micro-sized aluminum dust and its flame propagation properties in 20 L spherical vessel [J]. Powder Technology, 2016, 301: 1299–1308. DOI: 10.1016/j.powtec.2016.08.012.
|






 下载:
下载: