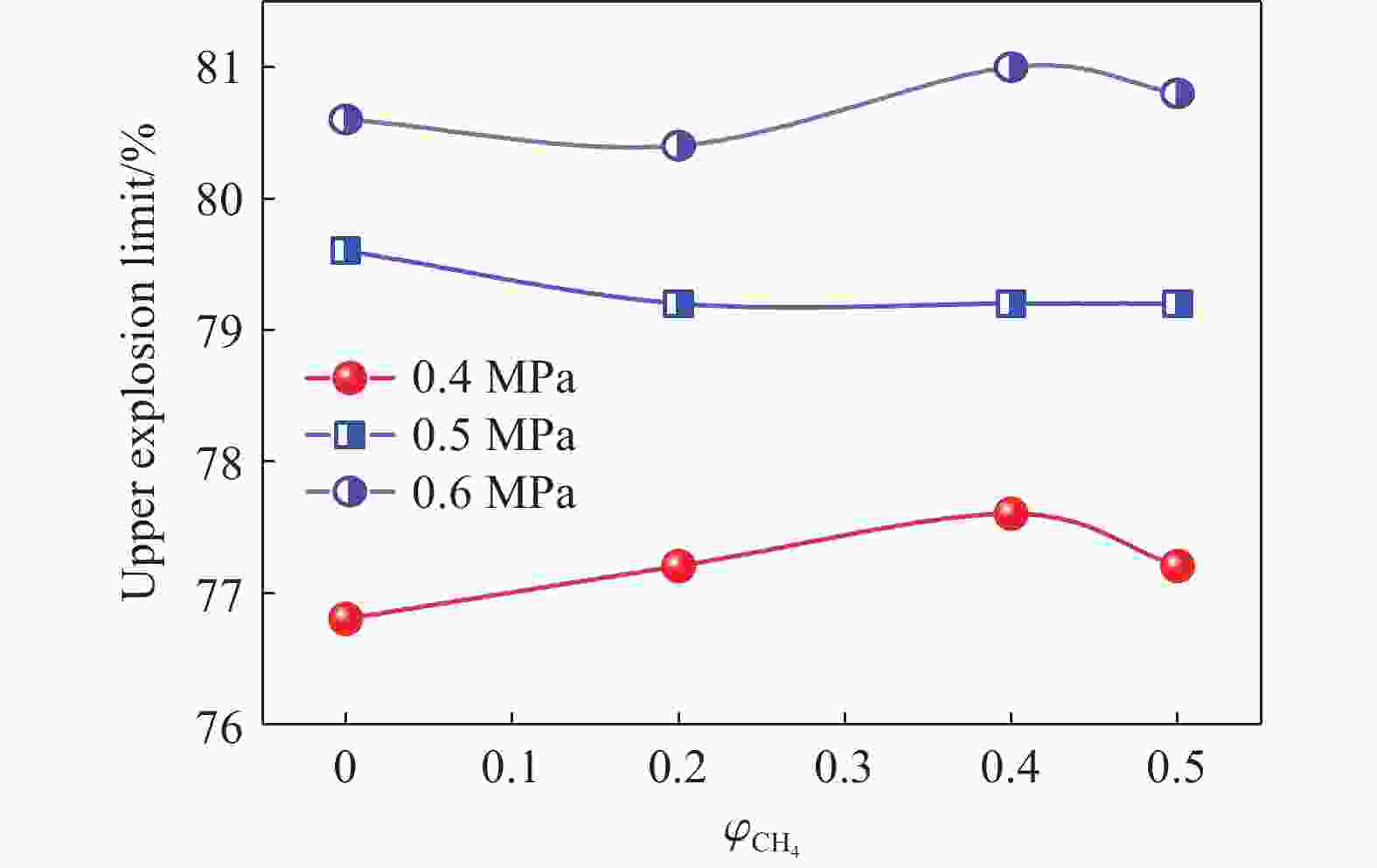
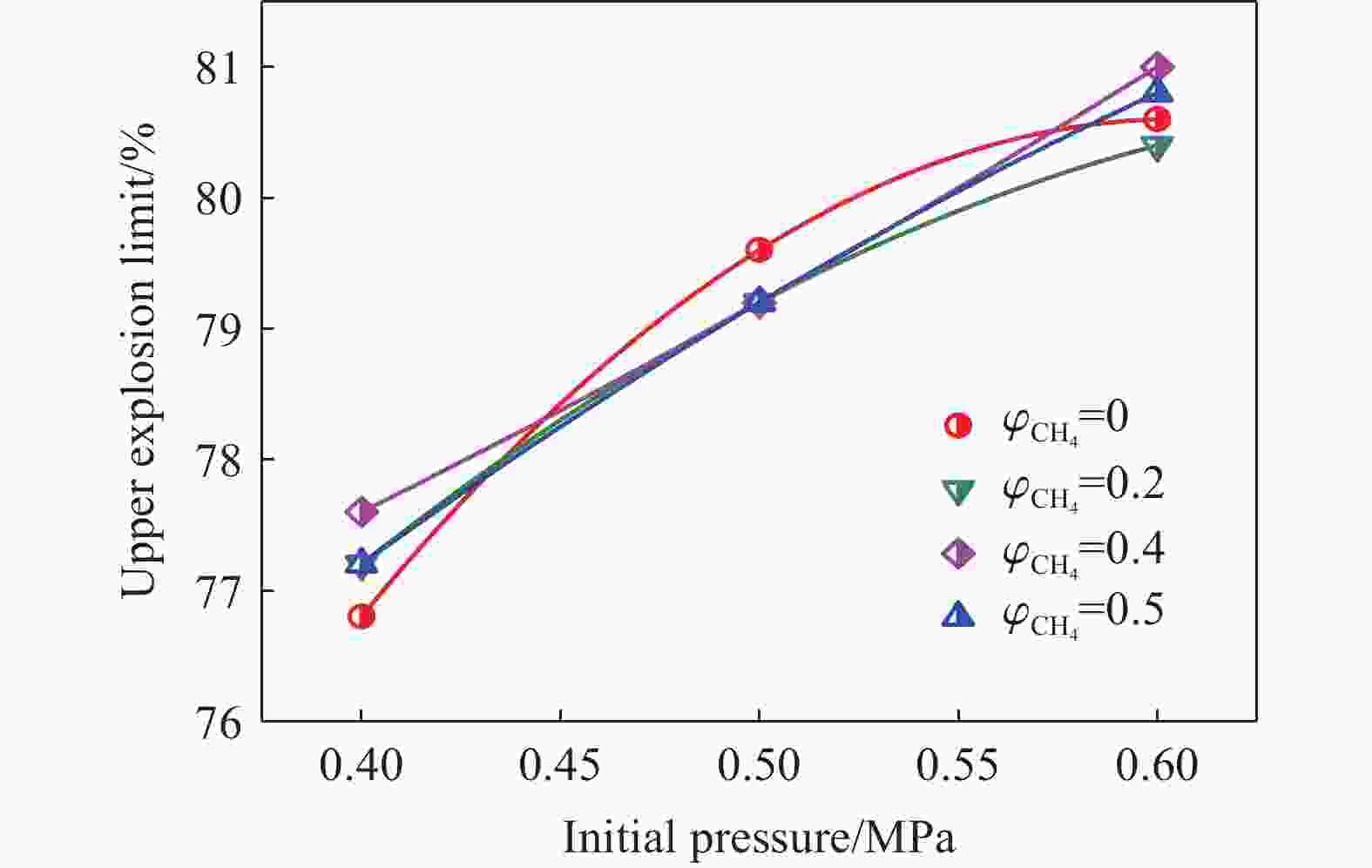
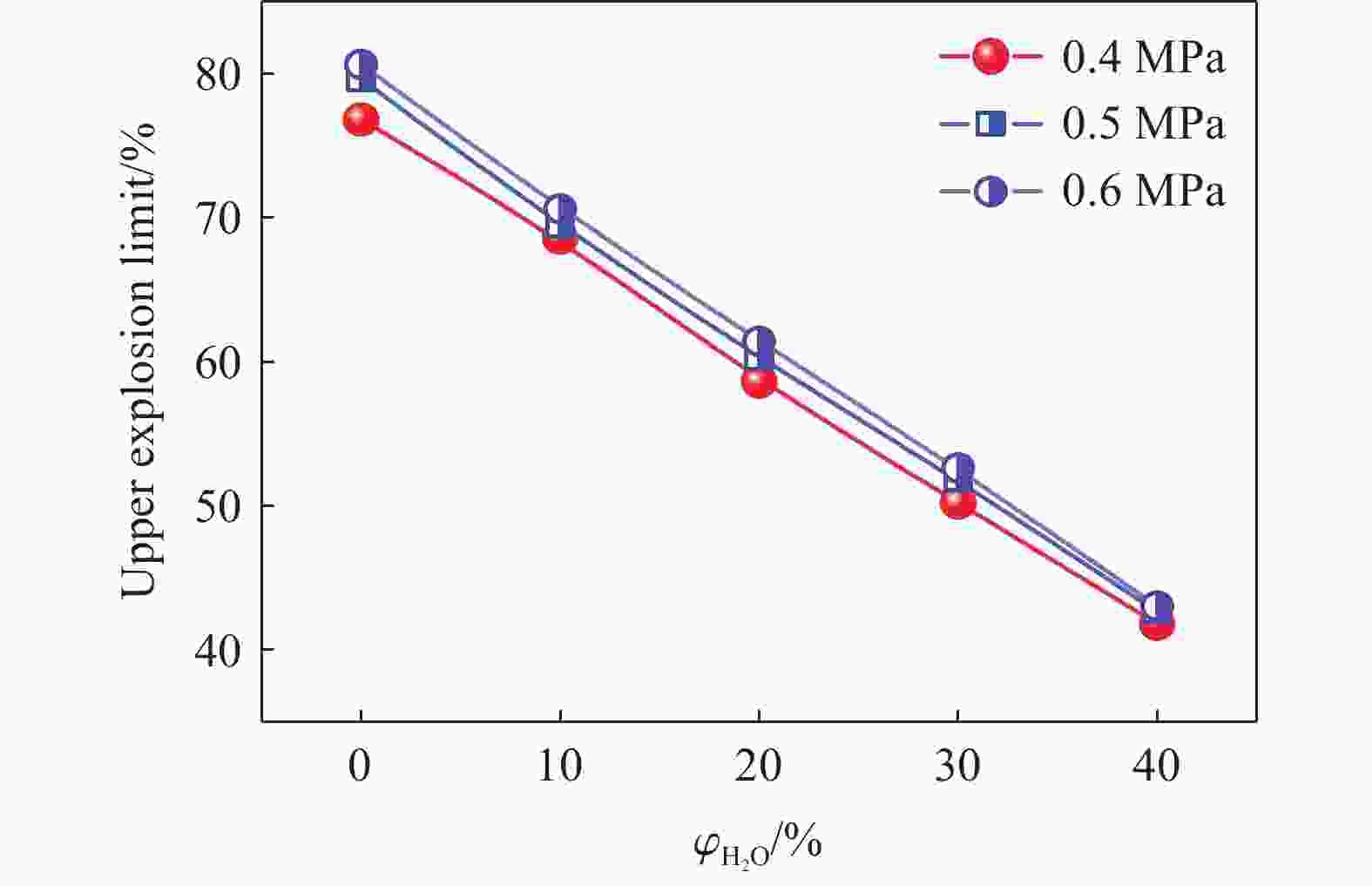
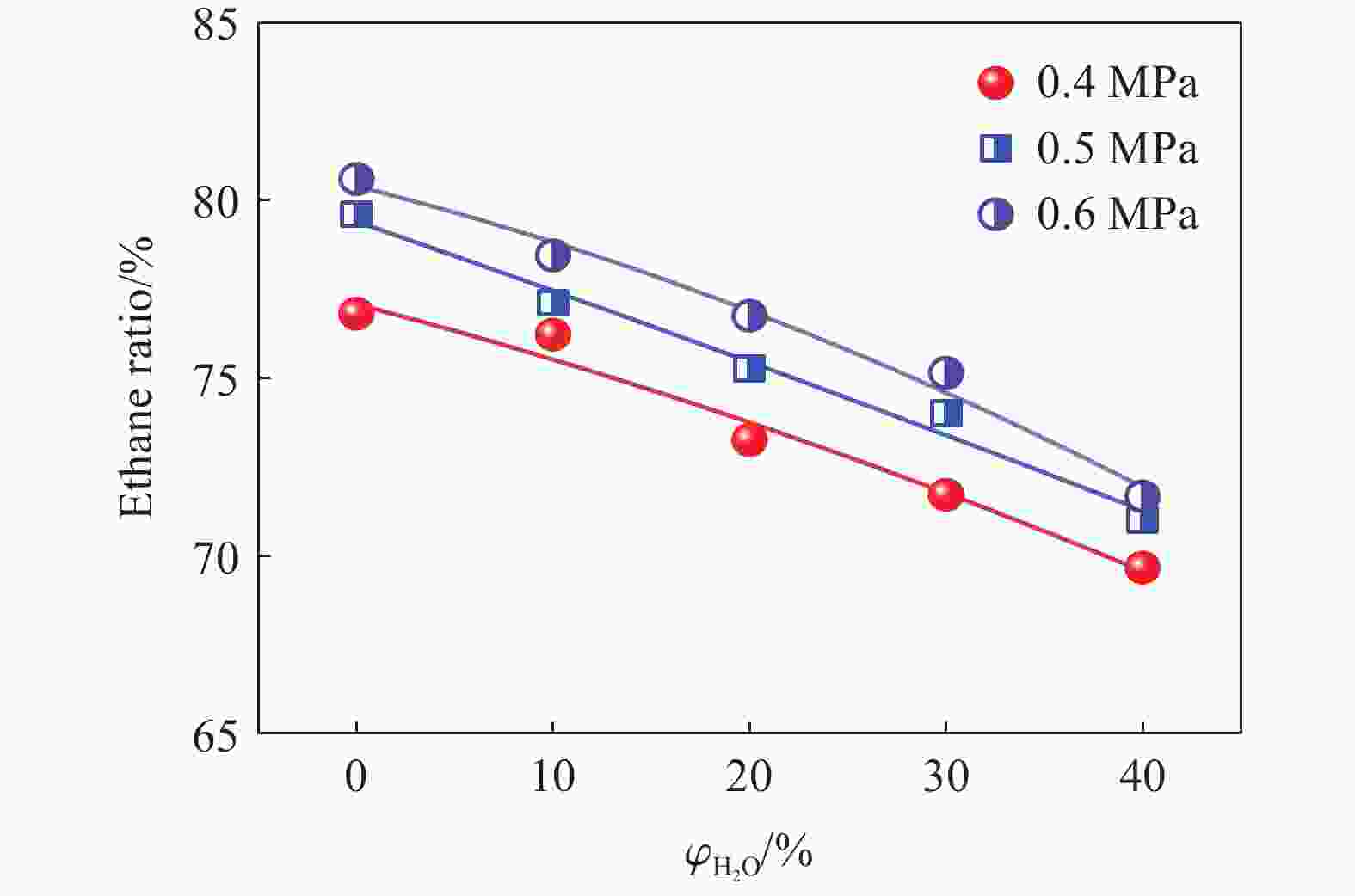
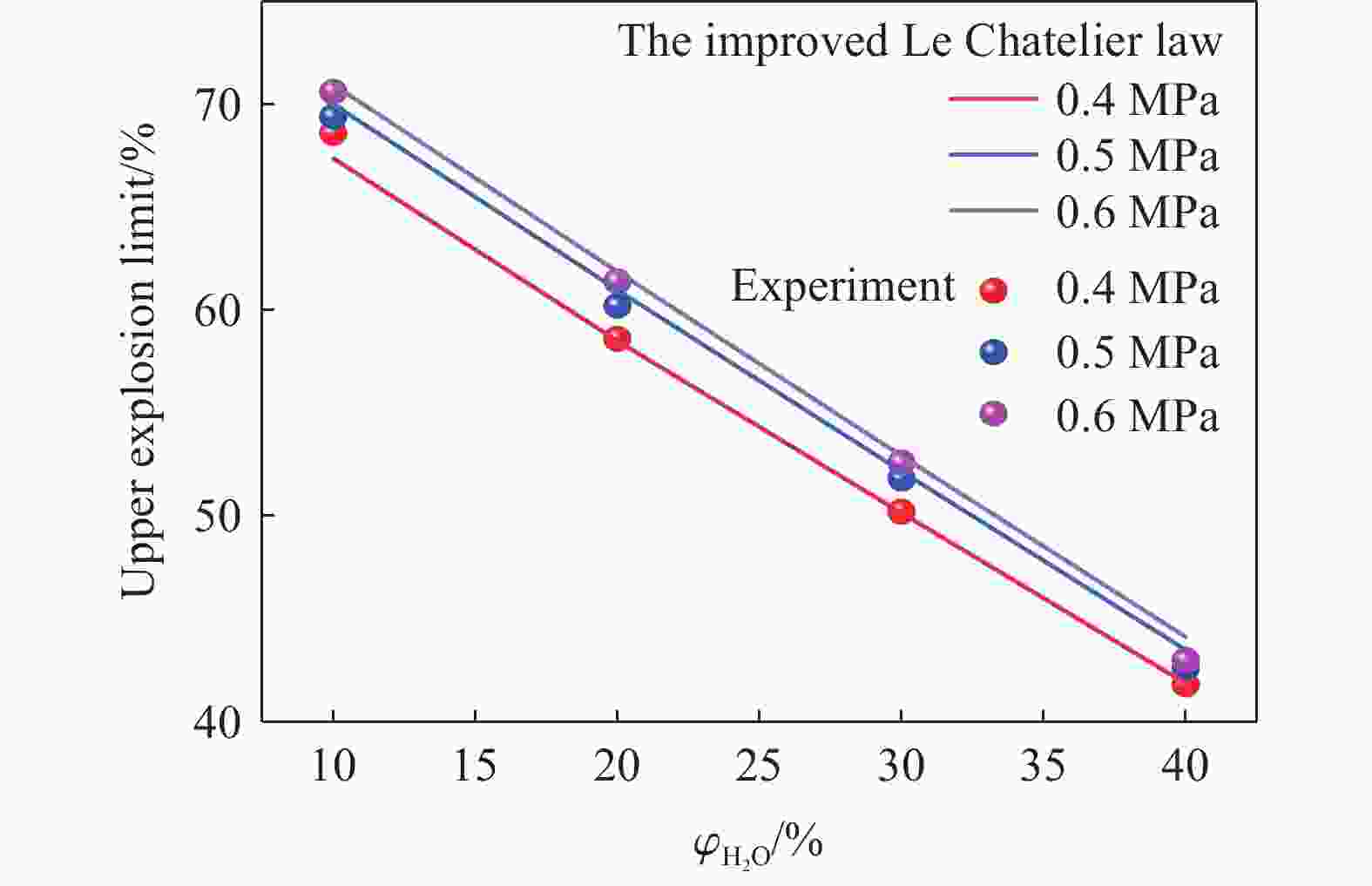
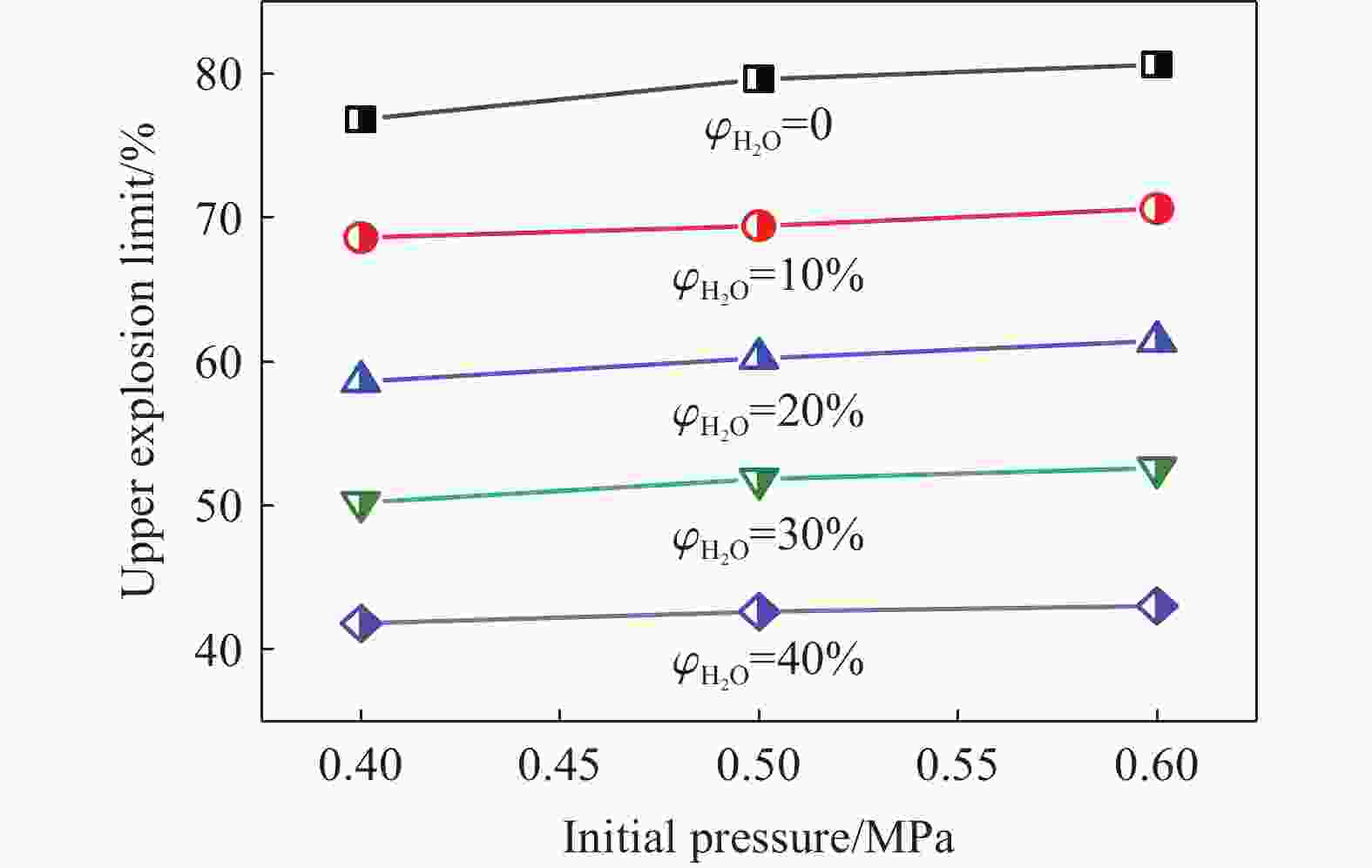
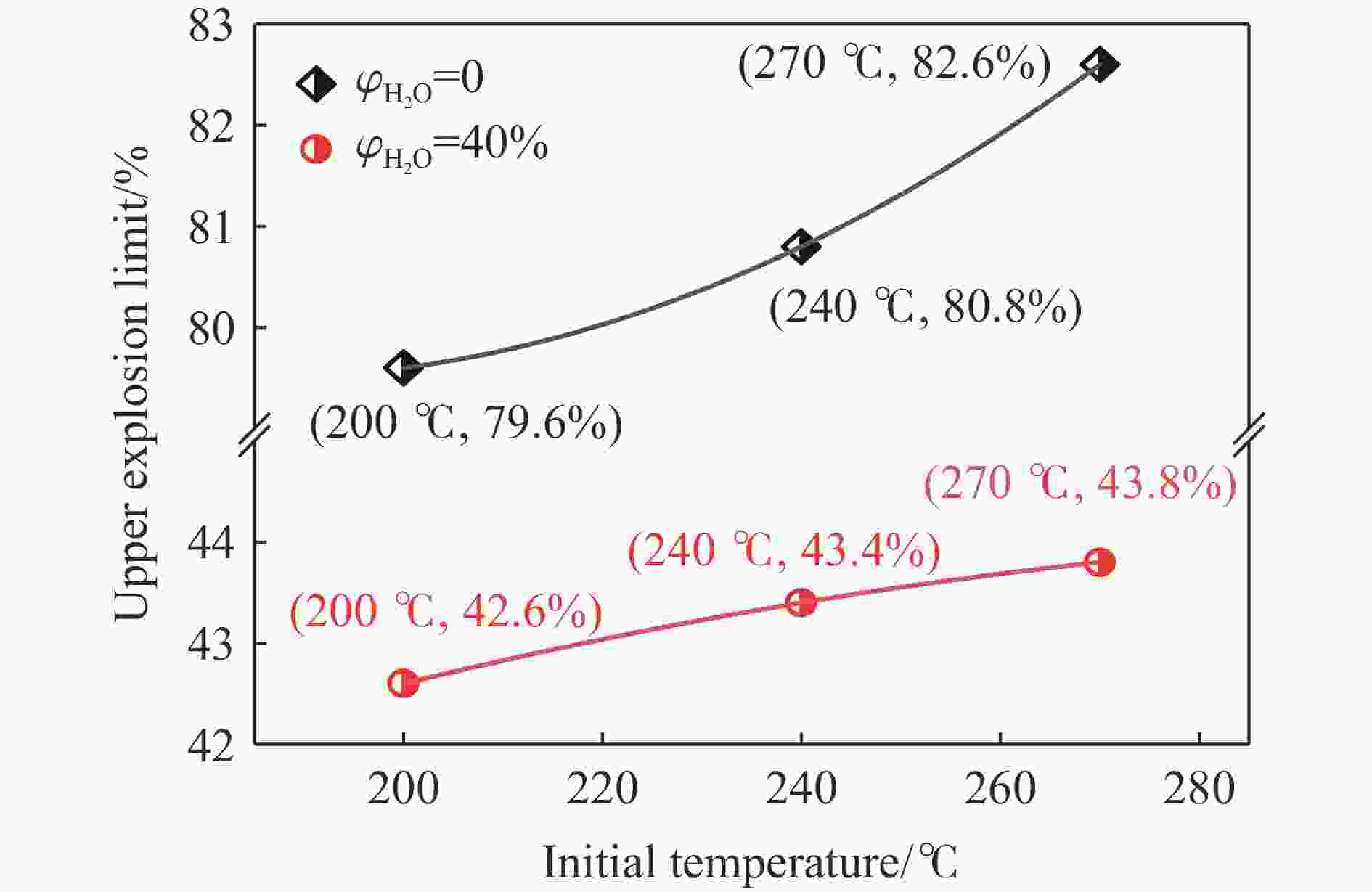
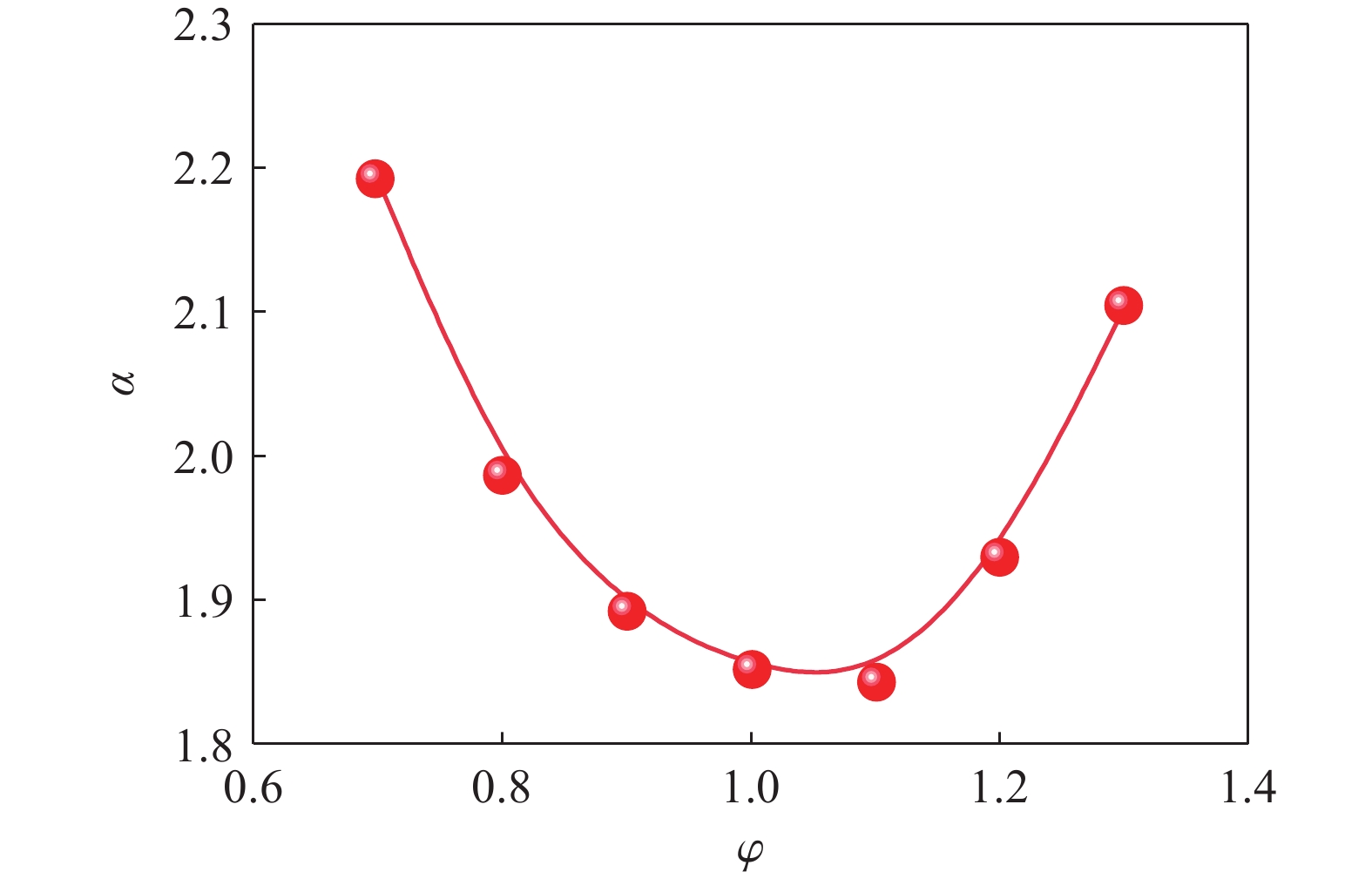
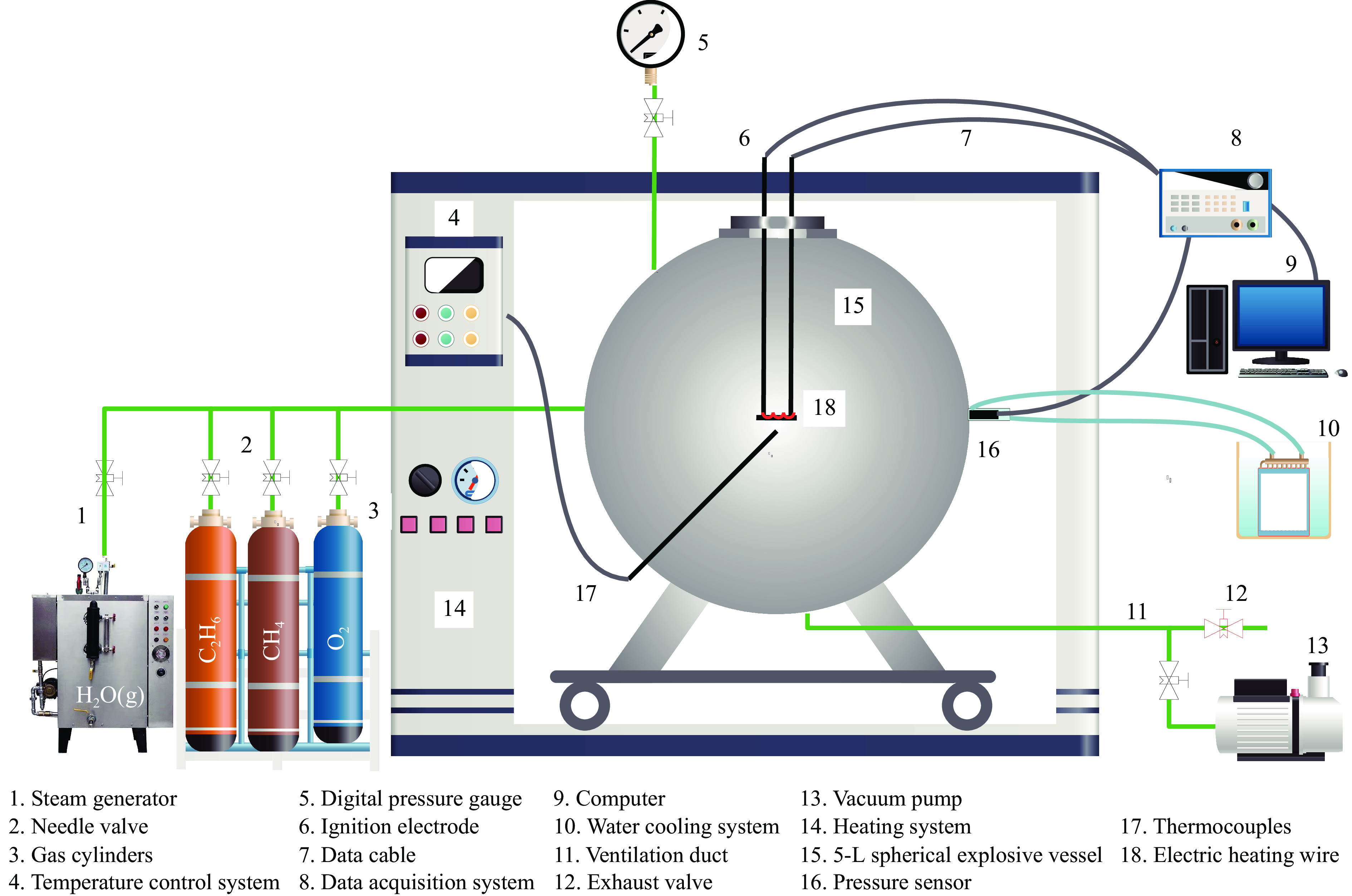
| [1] |
QI C, DING J F, WANG Y L, et al. Investigation of the upper flammability limit of ethylene/propane mixtures in air at high temperatures and pressures [J]. Energy, 2023, 281: 128114. DOI: 10.1016/j.energy.2023.128114.
|
| [2] |
QI C, WANG Y L, NING Y, et al. Investigation on the upper flammability limits of ethylene/air and propane/air mixtures at high temperature and pressure [J]. Journal of the Energy Institute, 2023, 109: 101291. DOI: 10.1016/j.joei.2023.101291.
|
| [3] |
QI C, YAN X Q, NING Y, et al. Investigation of the ignition temperature of ethane-air mixtures at elevated pressure [J]. Fuel, 2023, 350: 128815. DOI: 10.1016/j.fuel.2023.128815.
|
| [4] |
VAN DEN SCHOOR F, VERPLAETSEN F. The upper explosion limit of lower alkanes and alkenes in air at elevated pressures and temperatures [J]. Journal of Hazardous Materials, 2006, 128(1): 1–9. DOI: 10.1016/j.jhazmat.2005.06.043.
|
| [5] |
费轶, 金满平, 张帆, 等. 初始压力对烃类物质爆炸极限的影响及其预测模型研究 [J]. 安全、健康和环境, 2013, 13(11): 31–33. DOI: 10.3969/j.issn.1672-7932.2013.11.011.FEI Y, JIN M P, ZHANG F, et al. The influence of initial pressure on explosion limit of hydrocarbons substance and study on its prediction model [J]. Safety, Health and Environment, 2013, 13(11): 31–33. DOI: 10.3969/j.issn.1672-7932.2013.11.011.
|
| [6] |
HUANG L J, WANG Y, PEI S F, et al. Effect of elevated pressure on the explosion and flammability limits of methane-air mixtures [J]. Energy, 2019, 186: 115840. DOI: 10.1016/j.energy.2019.07.170.
|
| [7] |
MENDIBURU A, CARVALHO J, JU Y. Flammability limits: a comprehensive review of theory, experiments, and estimation methods[J]. Energy and Fuels, 2023. DOI: 10.1021/acs.energyfuels.2c03598.
|
| [8] |
YU X Z, YAN X Q, JI W T, et al. Effect of super-ambient conditions on the upper explosion limit of ethane/oxygen and ethylene/oxygen mixtures [J]. Journal of Loss Prevention in the Process Industries, 2019, 59: 100–105. DOI: 10.1016/j.jlp.2019.03.009.
|
| [9] |
QI C, YU X Z, WANG Y L, et al. Investigating the effect of temperature, pressure, and inert gas on the flammability range of ethane/oxygen mixtures [J]. Fuel, 2023, 354: 129296. DOI: 10.1016/j.fuel.2023.129296.
|
| [10] |
SONG D E, HU X Z. Effects of CO2 on the flammability limits of ethane in O2/CO2 atmosphere [J]. Fuel, 2022, 324: 124543. DOI: 10.1016/j.fuel.2022.124543.
|
| [11] |
ASTM International. Standard practice for determining limits of flammability of chemicals at elevated temperature and pressure: ASTM E918 [S]. West Conshohocken, PA: ASTM International, 2019.
|
| [12] |
British Standards Institution. Determination of explosion limits of gases and vapours at elevated pressures, elevated temperatures or with oxidizers other than air: EN 17624 [S]. London: British Standards Institution, 2022.
|
| [13] |
国家市场监督管理总局、国家标准化管理委员会. 高温高压条件下可燃气体(蒸气)爆炸极限测定方法: GB/T 42368—2023 [S]. 北京: 中国标准出版社, 2023.State Administration for Market Regulation, National Standardization Administration. Determination of explosion limits of combustible vapors and gases at elevated temperature and pressure: GB/T 42368—2023 [S]. Beijing: China Standard Press, 2023.
|
| [14] |
ZHANG B, CHANG X Y, BAI C H. End-wall ignition of methane-air mixtures under the effects of CO2/Ar/N2 fluidic jets [J]. Fuel, 2020, 270: 117485. DOI: 10.1016/j.fuel.2020.117485.
|
| [15] |
LI P, LI M Z, LIU Z Y, et al. Effect of high temperature and sulfur vapor on the flammability limit of hydrogen sulfide [J]. Journal of Cleaner Production, 2022, 337: 130579. DOI: 10.1016/j.jclepro.2022.130579.
|
| [16] |
DAVIS S G, LAW C K. Determination of and fuel structure effects on laminar flame speeds of C1 to C8 hydrocarbons [J]. Combustion Science and Technology, 1998, 140(1): 427–449. DOI: 10.1080/00102209808915781.
|
| [17] |
LEHN F, CAI L, PITSCH H. Impact of thermochemistry on optimized kinetic model predictions: auto-ignition of diethyl ether [J]. Combustion and Flame, 2019, 210: 454–466. DOI: 10.1016/j.combustflame.2019.09.011.
|
| [18] |
喻健良, 姚福桐, 于小哲, 等. 高温和高压对乙烷在氧气中爆炸极限影响的实验研究 [J]. 爆炸与冲击, 2019, 39(12): 122101. DOI: 10.11883/bzycj-2018-0381.YU J L, YAO F T, YU X Z, et al. Experimental study on the influence of high temperature and high pressure on the upper limit of explosion of ethane in oxygen [J]. Explosion and Shock Waves, 2019, 39(12): 122101. DOI: 10.11883/bzycj-2018-0381.
|
| [19] |
GU X J, HAQ M Z, LAWES M, et al. Laminar burning velocity and Markstein lengths of methane-air mixtures [J]. Combustion and Flame, 2000, 121(1/2): 41–58. DOI: 10.1016/S0010-2180(99)00142-X.
|
| [20] |
HASSAN M I, AUNG K T, FAETH G M. Measured and predicted properties of laminar premixed methane/air flames at various pressures [J]. Combustion and Flame, 1998, 115(4): 539–550. DOI: 10.1016/S0010-2180(98)00025-X.
|
| [21] |
JOMAAS G, ZHENG X L, ZHU D L, et al. Experimental determination of counterflow ignition temperatures and laminar flame speeds of C2–C3 hydrocarbons at atmospheric and elevated pressures [J]. Proceedings of the Combustion Institute. International Symposium on Combustion, 2005, 30(1): 193–200. DOI: 10.1016/j.proci.2004.08.228.
|
| [22] |
LOWRY W, DE VRIES J, KREJCI M, et al. Laminar flame speed measurements and modeling of pure alkanes and alkaneblends at elevated pressures [J]. Journal of engineering for gas turbines and power, 2011, 133(9). DOI: 10.1115/1.4002809.
|
| [23] |
GOSWAMI M, BASTIAANS R J M, DE GOEY L P H, et al. Experimental and modelling study of the effect of elevated pressure on ethane and propane flames [J]. Fuel, 2016, 166: 410–418. DOI: 10.1016/j.fuel.2015.11.013.
|
| [24] |
SHANG R, ZHUANG Z, NIU J, et al. Experimental study on the lower flammability limit of N2 and CO2 diluted H2/CO/air mixtures at high initial pressure [J]. International Journal of Hydrogen Energy, 2023, 48(1): 393–406. DOI: 10.1016/j.ijhydene.2022.09.255.
|
| [25] |
KONDO S, TAKIZAWA K, TAKAHASHI A, et al. Extended Le Chatelier’s formula and nitrogen dilution effect on the flammability limits [J]. Fire Safety Science, 2006, 41(5): 406–417. DOI: 10.1016/j.firesaf.2006.03.002.
|
| [26] |
KONDO S, TAKIZAWA K, TAKAHASHI A, et al. Extended Le Chatelier’s formula for carbon dioxide dilution effect on flammability limits [J]. Journal of Hazardous Materials, 2006, 138(1): 1–8. DOI: 10.1016/j.jhazmat.2006.05.035.
|
| [27] |
SHINDE V, FULZELE A, KUMAR S. Investigation on laminar burning velocity measurements of premixed ethane-air mixture at higher pressure and temperature conditions [J]. Fuel, 2024, 358: 130175. DOI: 10.1016/j.fuel.2023.130175.
|






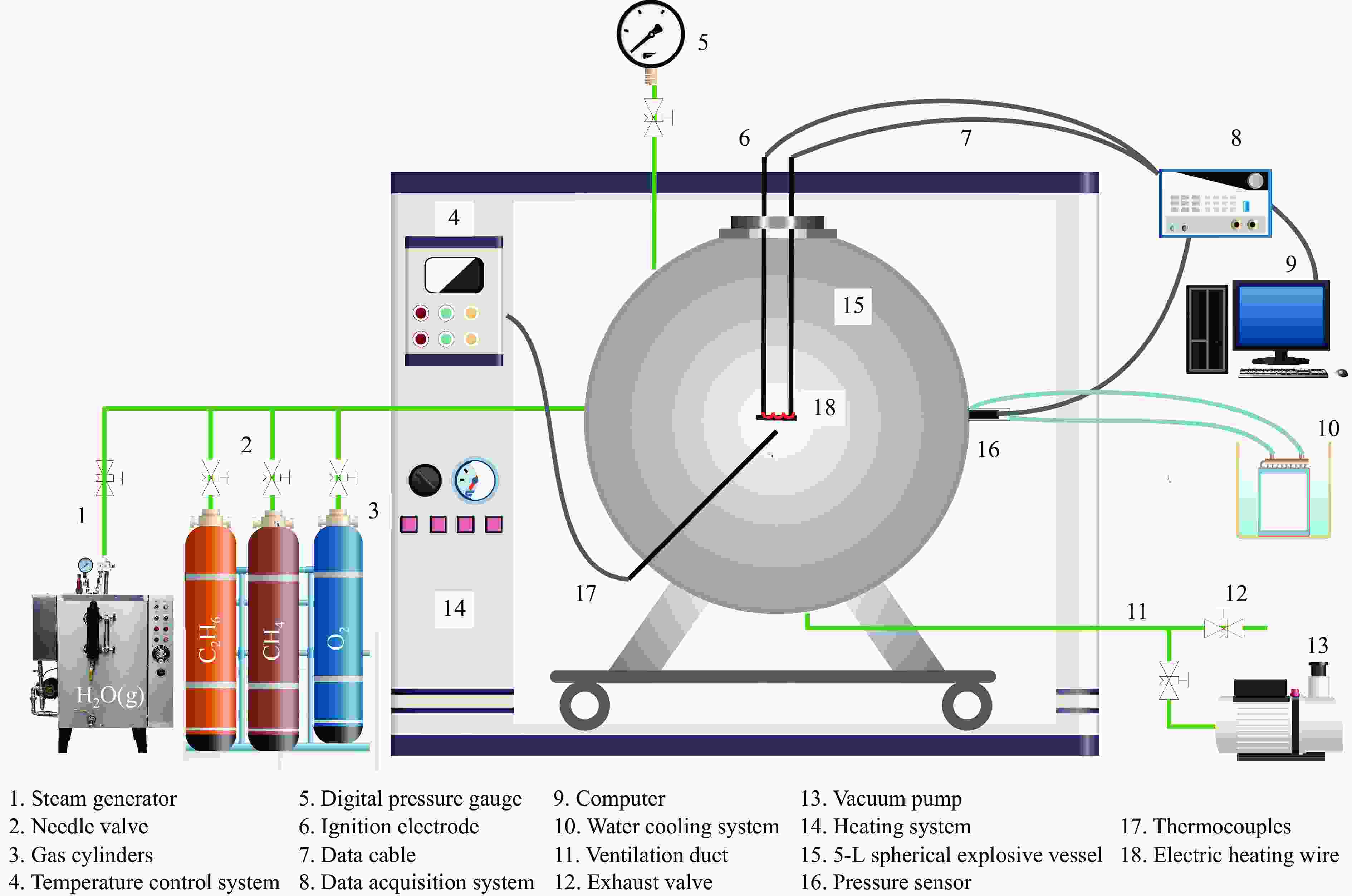
 下载:
下载: