Effects of hydrogen doping ratio and CO2 on the explosion characteristics of hydrogen-doped natural gas
-
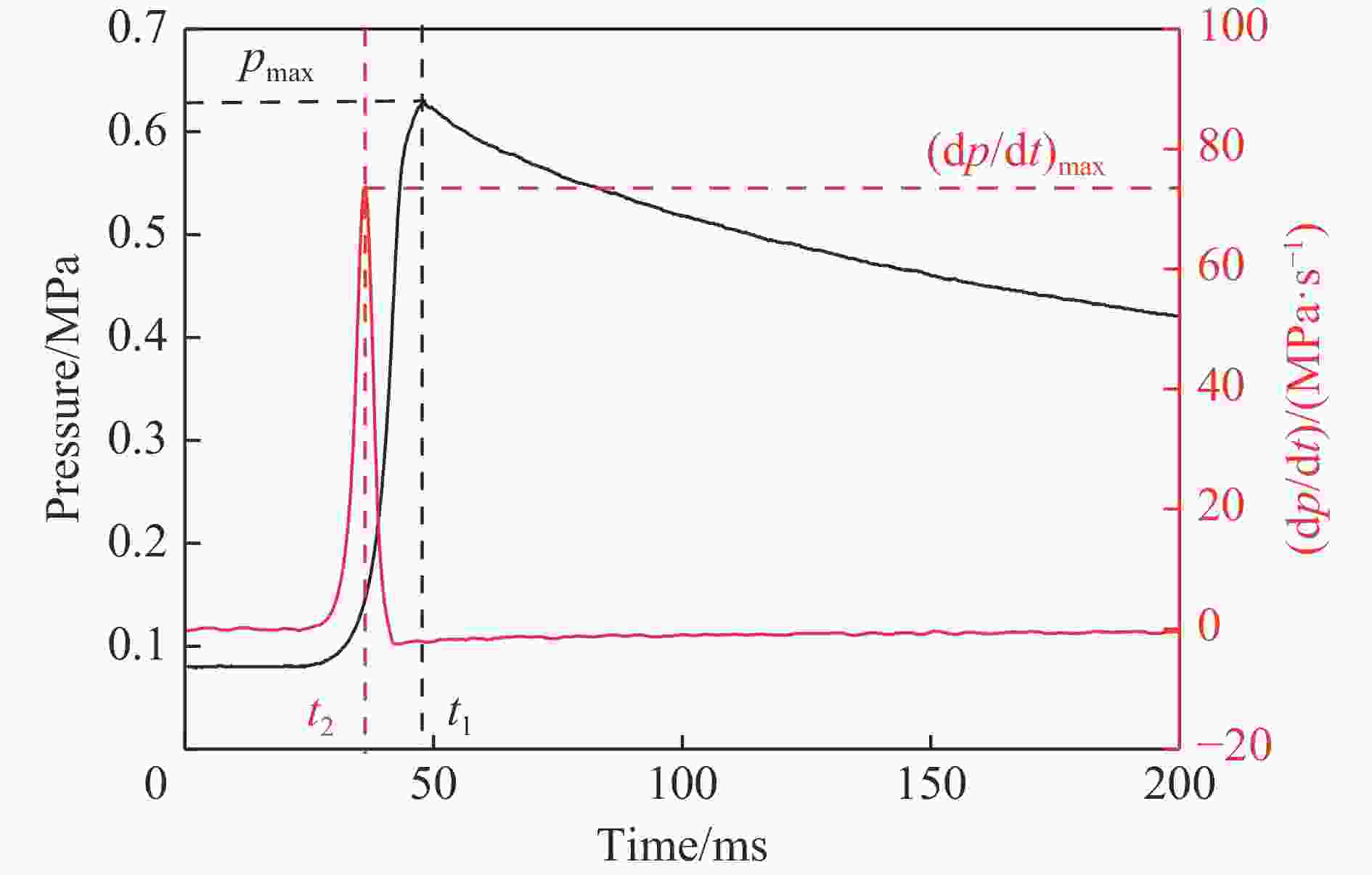
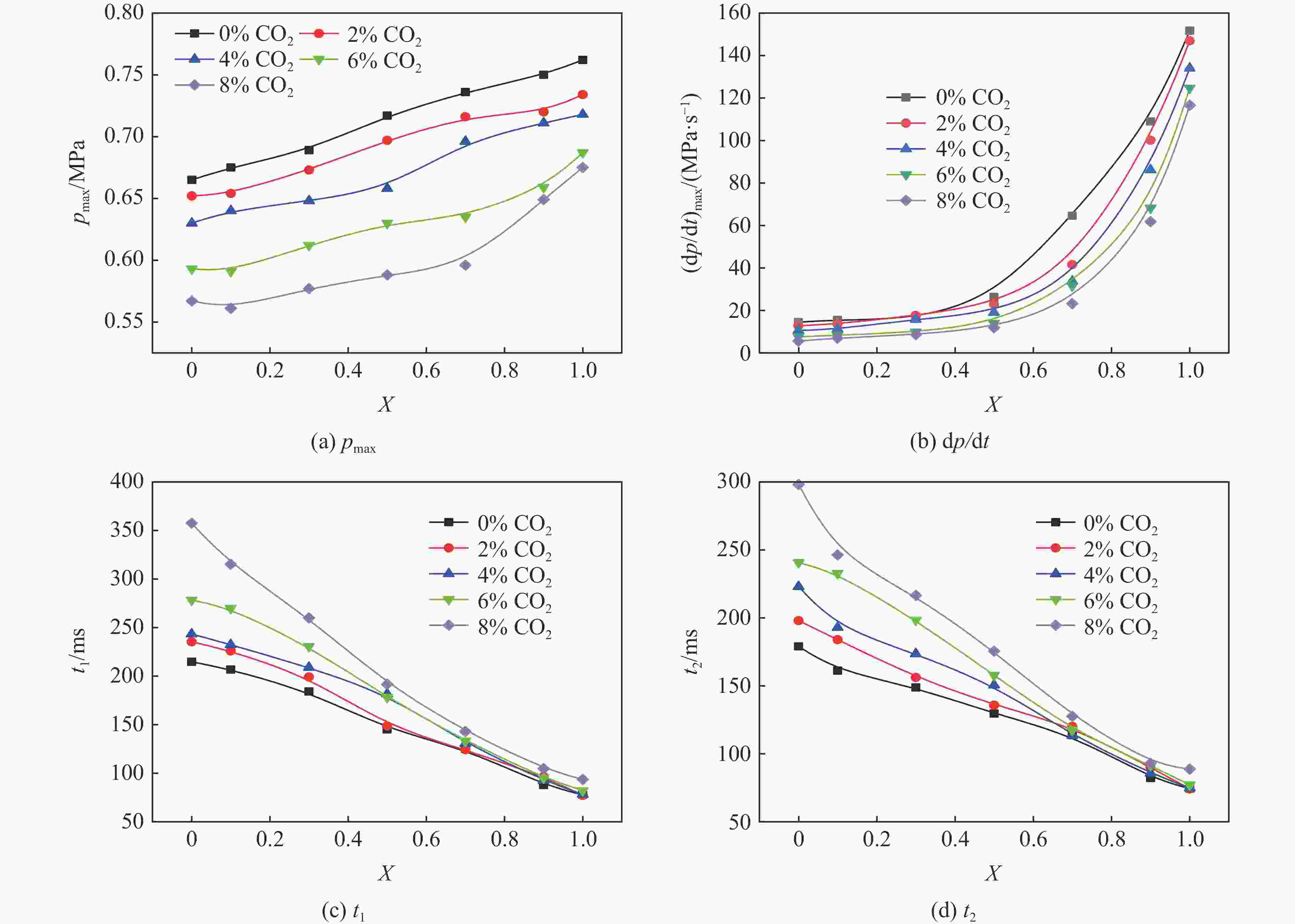
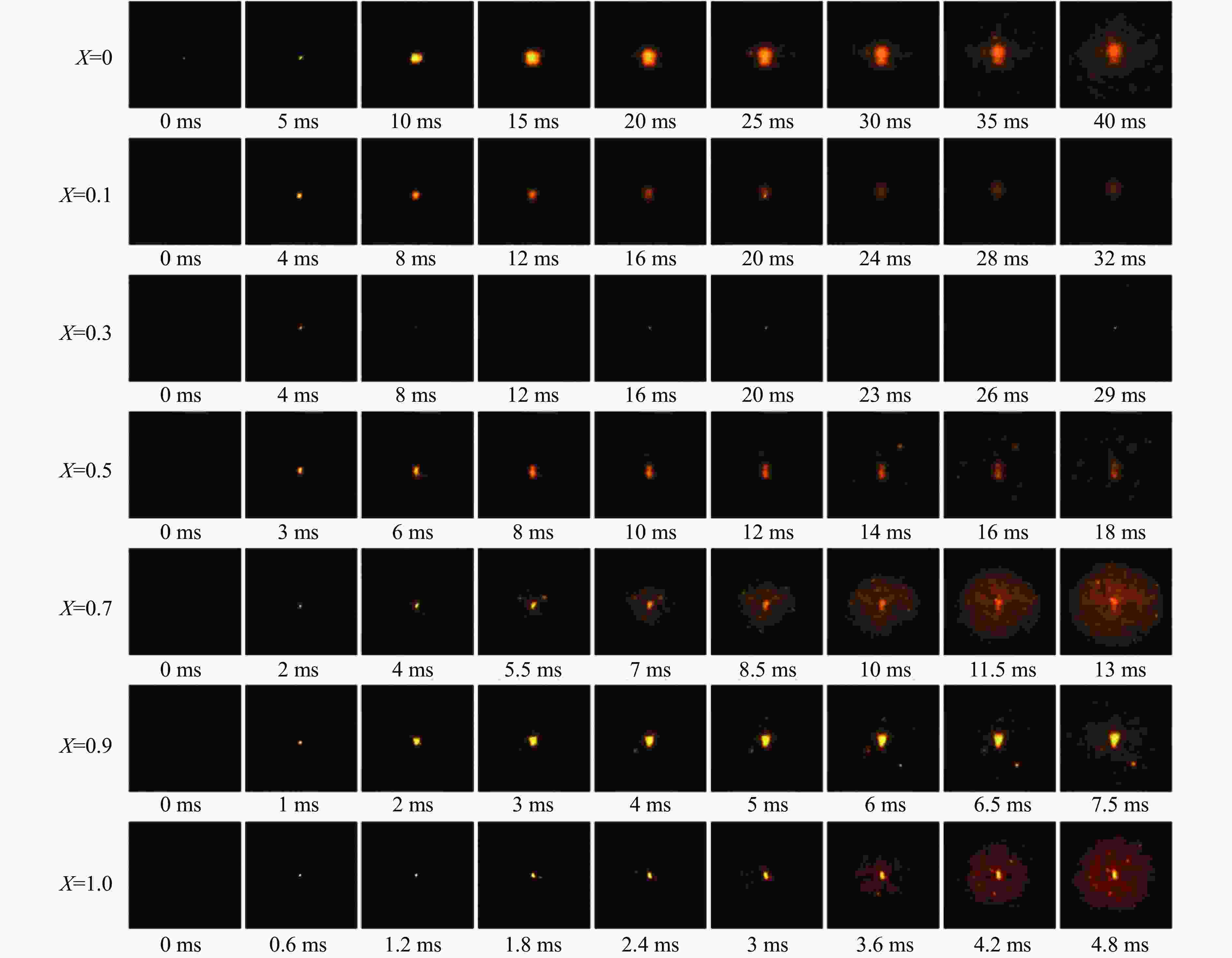
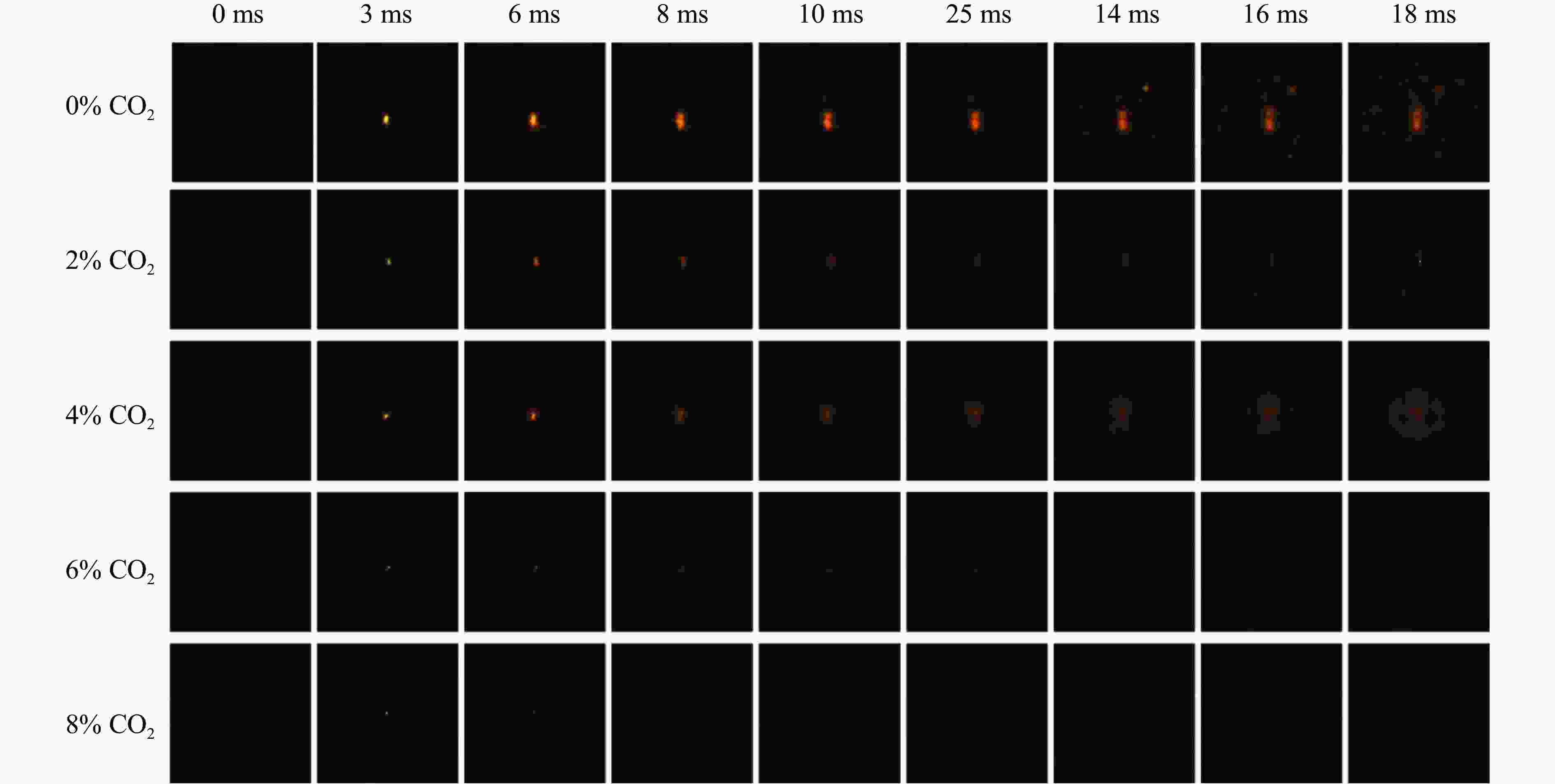
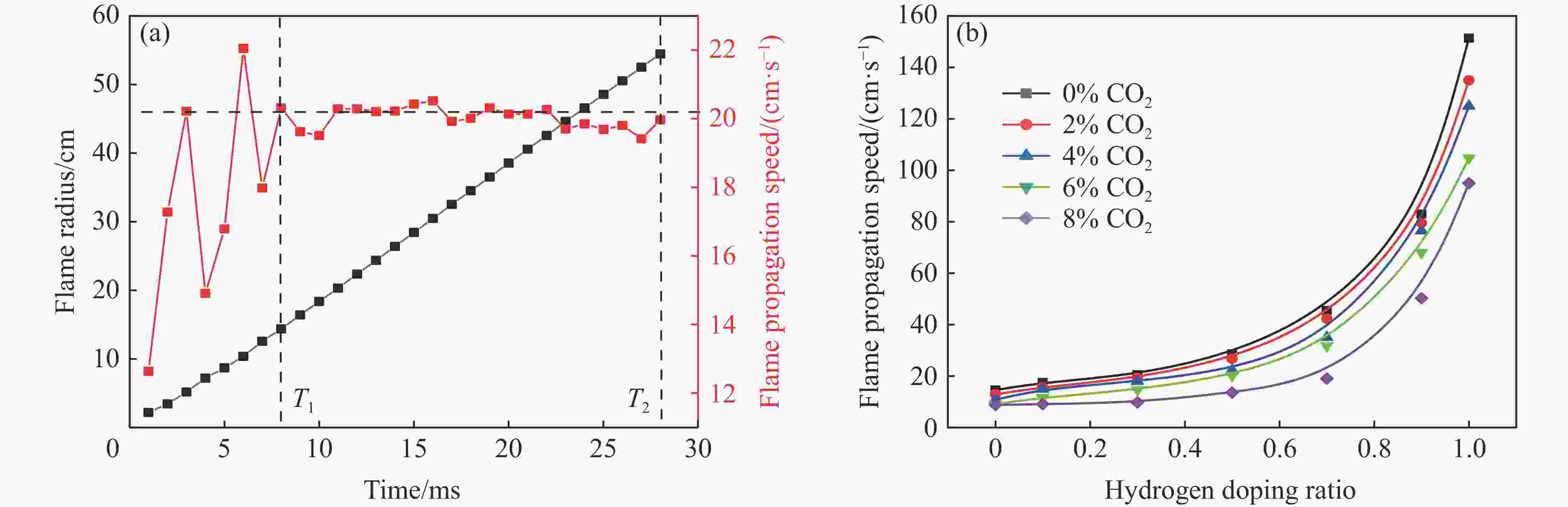
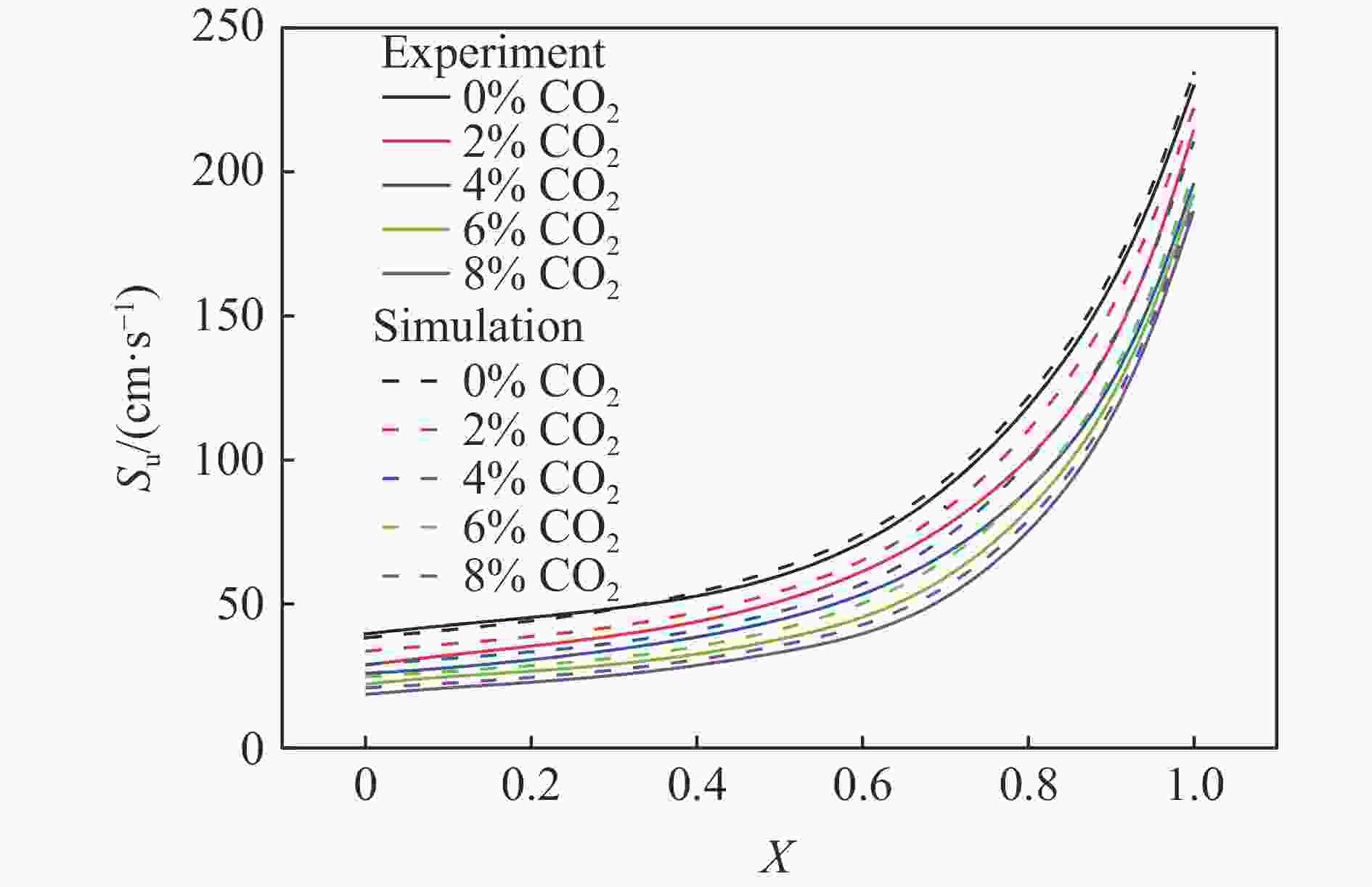
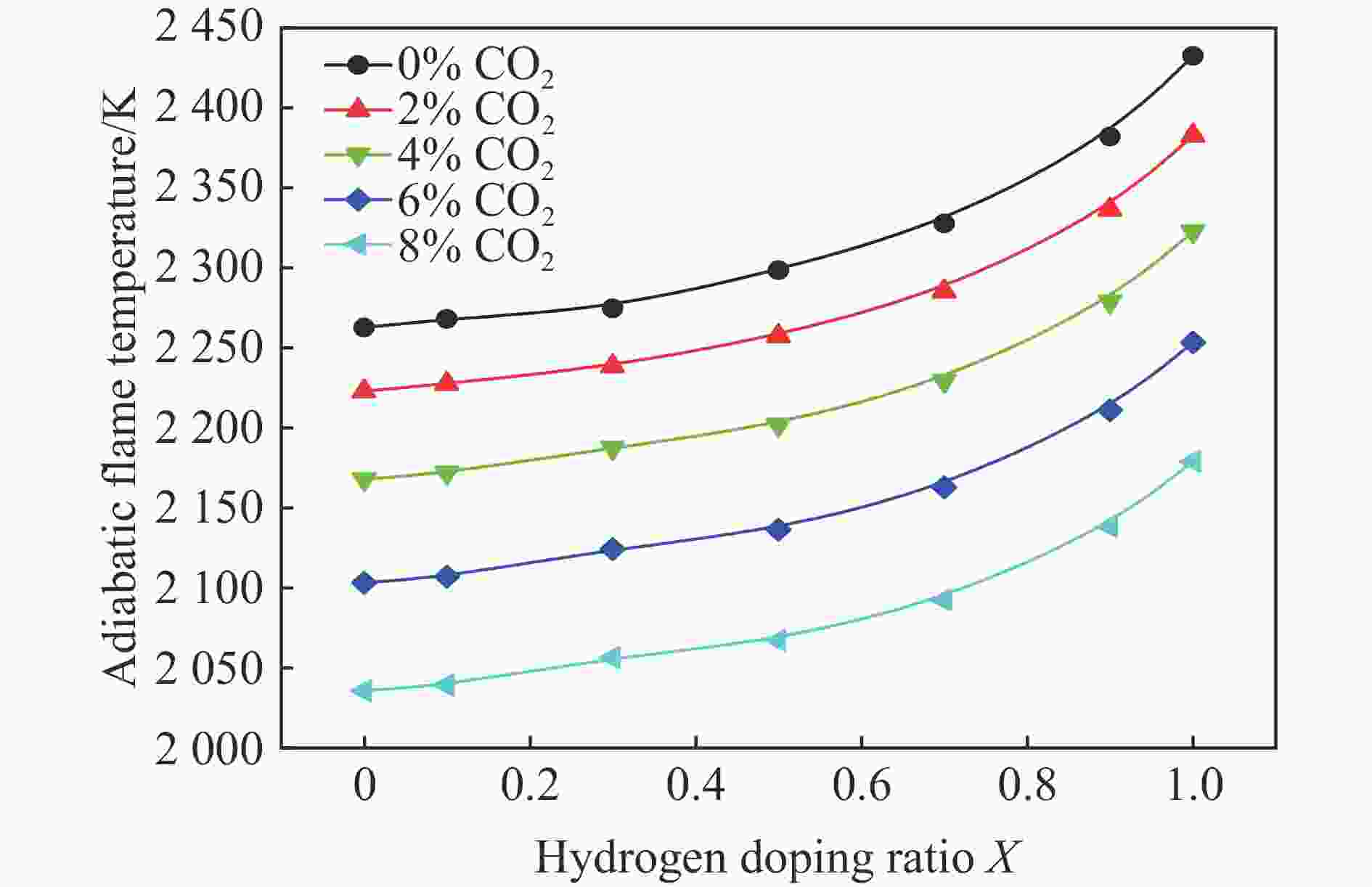
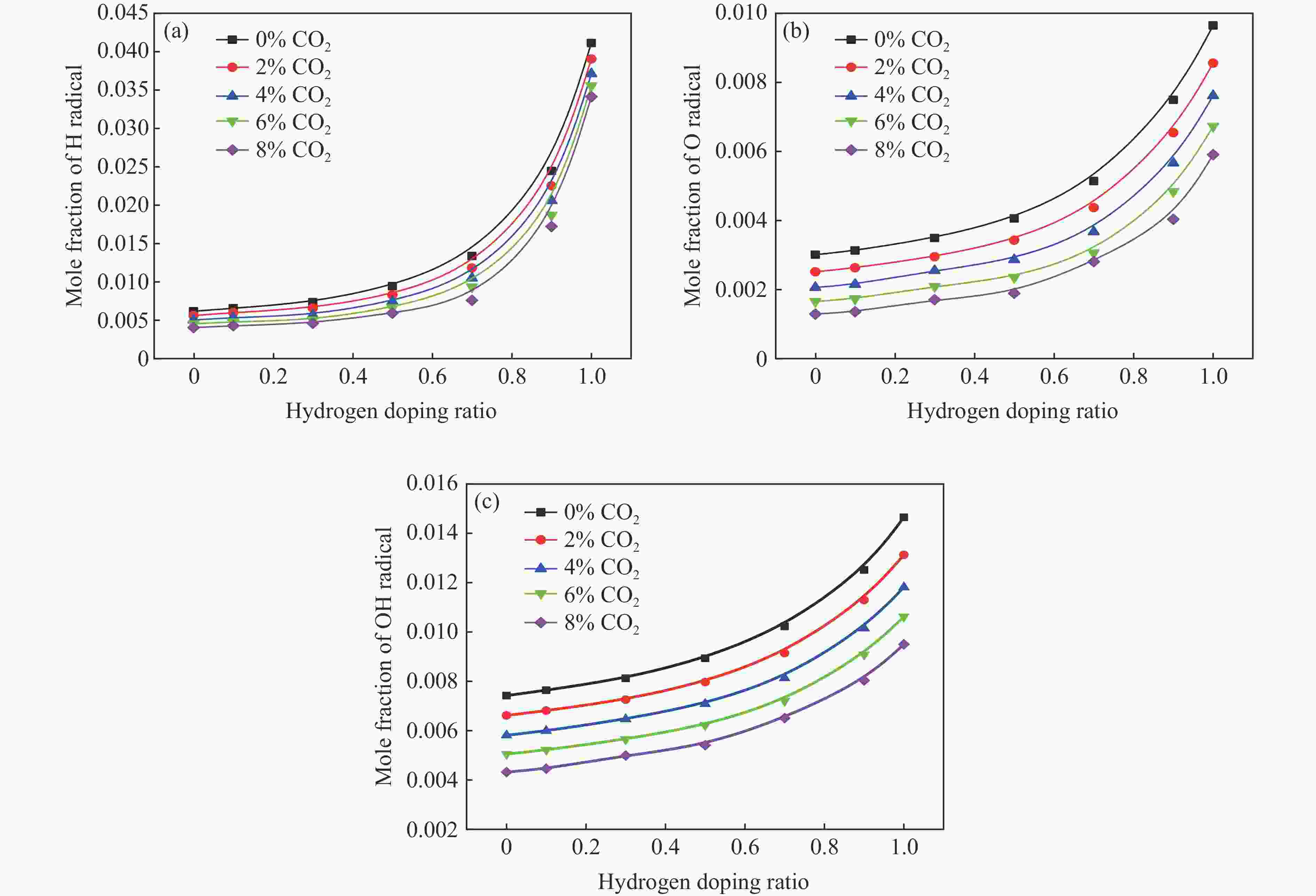
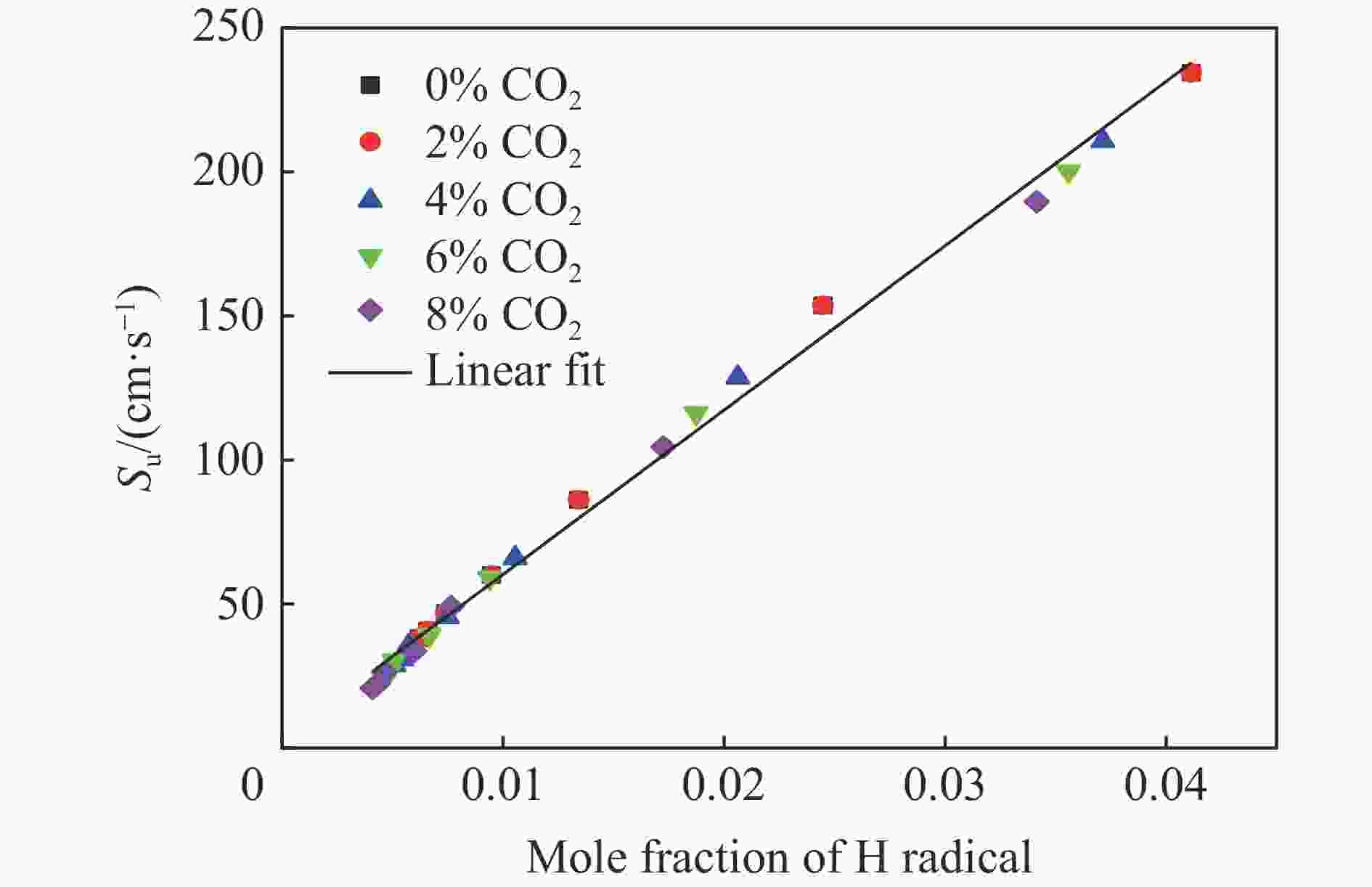
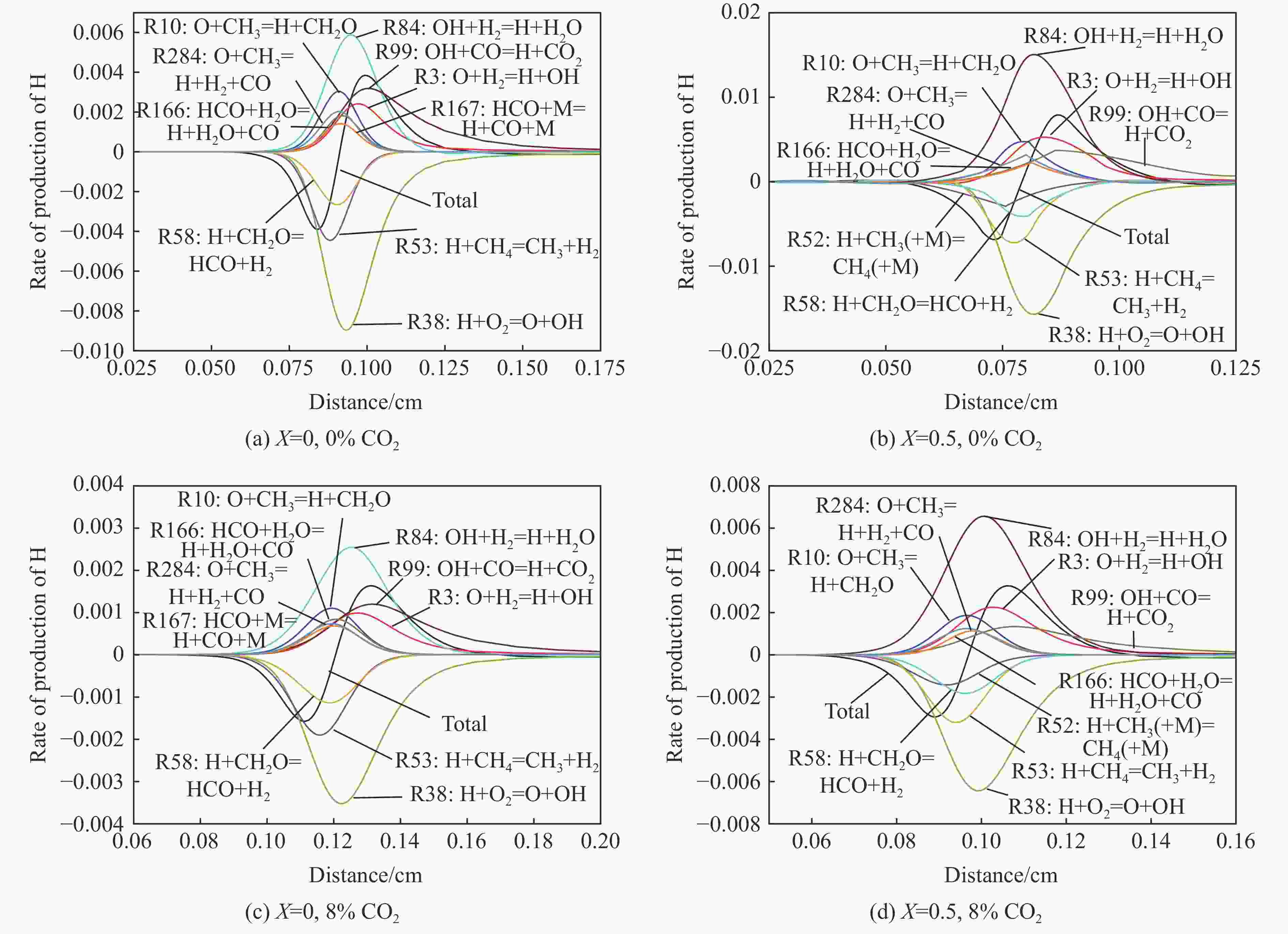
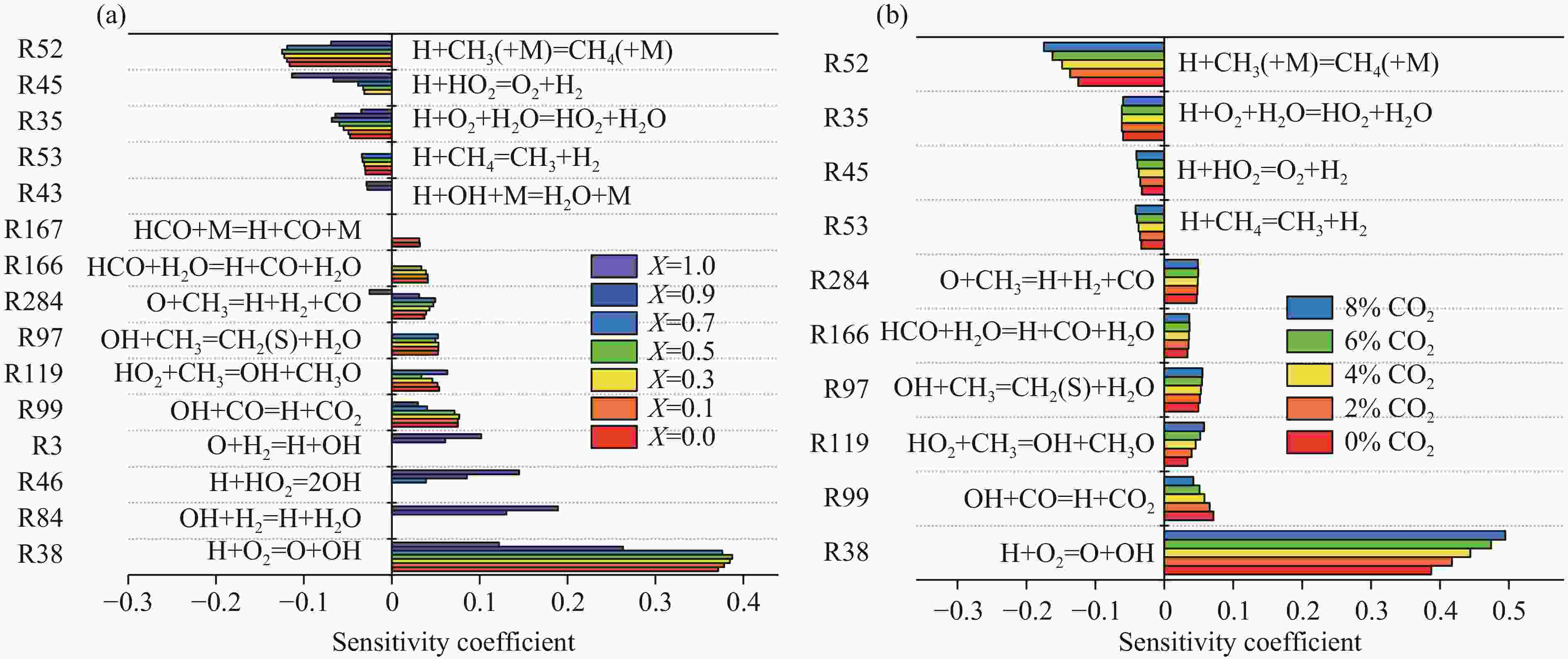
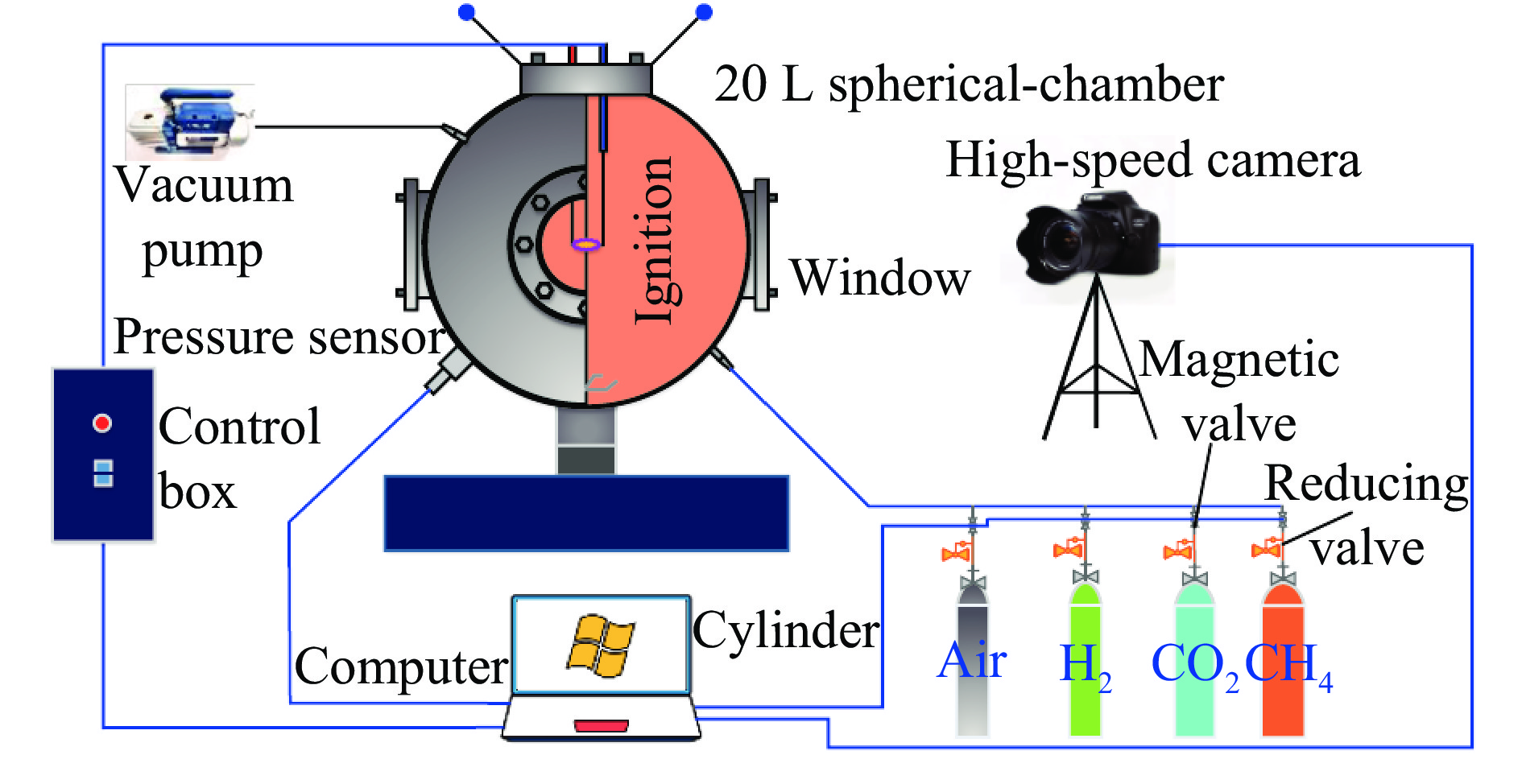
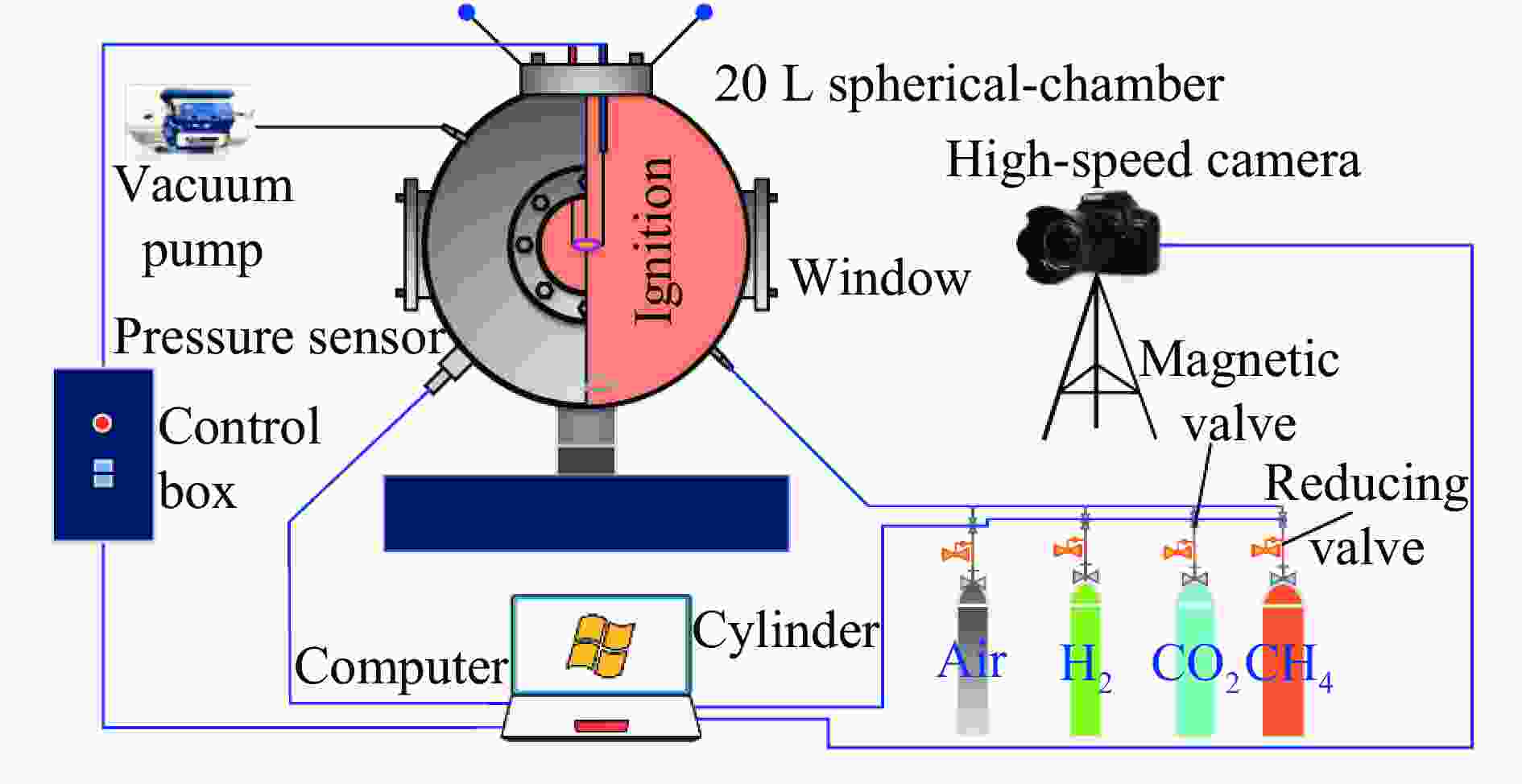
摘要: 采用20 L球形装置研究了掺氢比和添加CO2对掺氢天然气爆炸压力和火焰传播特性的影响。结果表明:掺氢比对掺氢天然气爆炸压力和火焰传播速度有促进作用。随着掺氢比的提高,最大爆炸压力逐渐上升,快速燃爆时间和持续燃烧时间缩短,最大爆炸压力上升速率和火焰传播速度在掺氢比小于0.5时逐渐上升,当掺氢比大于0.5时,最大爆炸压力上升速率和火焰传播速度快速上升。加入CO2对混合气体爆炸压力和火焰传播速度有抑制作用,但对高掺氢比天然气的压力参数抑制效果较差。通过反应动力学分析可知,随着掺氢比的提高,火焰层流燃烧速度和绝热火焰温度逐渐上升,活性自由基摩尔分数和产物生成速率明显上升,并且掺混氢气改变了甲烷的反应路径,当掺氢比大于0.5时,反应R84、R46和R3进入了前10步反应中,产生了H和OH自由基,促进了反应。而CO2能降低混合气体的层流燃烧速率、绝热火焰温度、活性自由基摩尔分数以及产物生成速率,但添加CO2不改变甲烷的反应路径。Abstract: Hydrogen-doped natural gas technology has been gradually used in pipeline transportation, but hydrogen-doped natural gas is suspectible to leakage and explosion accidents. The study used a 20-L spherical device to investigate the effects of hydrogen doping ratio and CO2 addition on the explosion pressure and flame propagation characteristics of hydrogen-doped natural gas, and the chemical reaction kinetics method was used to analyze the explosion mechanism. The results showed that the hydrogen doping ratio has a promoting effect on the pressure parameters of hydrogen-doped natural gas explosion and flame propagation speed. As the hydrogen doping ratio increases, the maximum explosion pressure gradually increases, while both the rapid burn time and sustained burn time decrease progressively. The maximum rise rate of explosion pressure and flame propagation speed gradually increase when the hydrogen doping ratio is lower than 0.5. When the hydrogen doping ratio is greater than 0.5, the maximum rise rate of explosion pressure and flame propagation speed rise rapidly. The addition of CO2 has an inhibitory effect on the explosion pressure and flame propagation speed of the mixed gas, but the suppression effect on pressure parameters with high hydrogen doping ratio is weak. Through reaction kinetic analysis, it can be seen that as the hydrogen doping ratio increases, the laminar burning velocity and adiabatic flame temperature gradually increase. Meanwhile the concentration of active free radicals and the product formation rate increase significantly, and the mixing of hydrogen changes the reaction path of methane. When the hydrogen doping ratio is greater than 0.5, reactions R84, R46 and R3 enter the top ten steps of the reaction, producing H and OH radicals, which promotes the reaction. CO2 can reduce the laminar burning velocity, adiabatic flame temperature, active free radical concentration and product formation raten of the mixed gas, but adding CO2 does not change the reaction path of methane.Hydrogen-doped natural gas technology has been gradually used in pipeline transportation, but hydrogen-doped natural gas is prone to leakage and explosion accidents. The study used a 20L spherical device to investigate the effects of hydrogen blending ratio and CO2 addition on the explosion pressure and flame propagation characteristics of hydrogen-doped natural gas, and the chemical reaction kinetics method was used to analyse the explosion mechanism. The results showed that the hydrogen doping ratio has a promoting effect on the hydrogen-doped natural gas explosion pressure parameters and flame propagation speed. As the hydrogen doping ratio increases, the maximum explosion pressure gradually increases, the rapid burn time and sustained burn time are gradually decreasing. The maximum explosion pressure rise rate and flame propagation speed gradually increase when the hydrogen doping ratio is less than 0.5. When the hydrogen doping ratio is greater than 0.5, the maximum explosion pressure rise rate and flame propagation speed rise rapidly. The addition of CO2 has an inhibitory effect on the explosion pressure and flame propagation speed of the mixed gas, but the suppression effect on pressure parameters with high hydrogen doping ratio is poor. Through reaction kinetic analysis, it can be seen that as the hydrogen doping ratio increases, the laminar burning velocity and adiabatic flame temperature gradually increase, the mole fraction of active free radicals and the rate of product increase significantly, and the mixing of hydrogen changes the reaction path of methane. When the hydrogen doping ratio is greater than 0.5, reactions R84, R46 and R3 enter the top ten steps of the reaction, producing H and OH radicals, which promotes the reaction. CO2 can reduce the laminar burning velocity, adiabatic flame temperature, active free radical mole fraction and rate of production of the mixed gas, but adding CO2 does not change the reaction path of methane.
-
Key words:
- gas explosion /
- hydrogen doping ratio /
- explosion suppression /
- kinetic analysis
-
表 1 掺氢比以及氢气和甲烷的体积分数
Table 1. Hydrogen doping ratio and volume fractions of hydrogen and methane
X ${x_{{{\text{H}}_{\text{2}}}}} $/% ${x_{{\text{C}}{{\text{H}}_{\text{4}}}}} $/% 0 0 9.50 0.1 1.02 9.18 0.3 3.36 8.36 0.5 7.19 7.19 0.7 12.67 5.43 0.9 21.98 2.44 1.0 29.59 0 -
[1] 李敬法, 苏越, 张衡, 等. 掺氢天然气管道输送研究进展 [J]. 天然气工业, 2021, 41(4): 137–152. DOI: 10.3787/j issn.1000-0976.2021.04.015.LI J F, SU Y, ZHANG H , et al. Research progresses on pipeline transportation of hydrogen-blended natural gas [J]. Natural Gas Industry, 2021, 41(4): 137–152. DOI: 10.3787/j issn.1000-0976.2021.04.015. [2] ZHOU S Y, XIAO J, LUO Z M, et al. Analysis of spontaneous ignition of hydrogen-enriched methane in a rectangular tube [J]. Proceedings of the Combustion Institute, 2024, 40(1): 105681. DOI: 10.1016/j.proci.2024.105681. [3] QU J, WANG R, DENG J, et al. Synergistic inhibition characteristics and kinetics of hydrogen explosion by two-phase suppressant N2-KHCO3 [J]. Journal of Loss Prevention in the Process Industries, 2024, 92: 105487. DOI: 10.1016/j.jlp.2024.105487. [4] WANG T, DONG Z, YANG P, et al. Phase change materials as hydrogen explosion suppressants: an experimental and kinetic investigation [J]. Chemical Engineering Journal, 2024, 500: 156996. DOI: 10.1016/j.cej.2024.156996. [5] MOLNARNE M, SCHROEDER V. Hazardous properties of hydrogen and hydrogen containing fuel gases [J]. Process Safety and Environmental Protection, 2019, 130: 1–5. DOI: 10.1016/j.psep.2019.07.012. [6] SHEN X B, XIU G L, WU S Z. Experimental study on the explosion characteristics of methane/air mixtures with hydrogen addition [J]. Applied Thermal Engineering, 2017, 120: 741–747. DOI: 10.1016/j.applthermaleng.2017.04.040. [7] SALZANO E, CAMMAROTA F, BENEDETTO A D, et al. Explosion behavior of hydrogen-methane/air mixtures [J]. Journal of Loss Prevention in the Process Industries, 2012, 25(3): 443–447. DOI: 10.1016/j.jlp.2011.11.010. [8] 董冰岩, 查裕学, 邹颖, 等. 球形压力容器中甲烷-氢气-空气爆炸过程数值模拟及实验研究 [J]. 中国安全生产科学技术, 2023, 19(3): 157–163. DOI: 10.11731/j.issn.1673-193x.2023.03.023.DONG B Y, CHA Y X, ZOU Y, et al. Numerical simulation and experimental study of methane-hydrogen-air explosion process in spherical pressure vessel [J]. Journal of Safety Science and Technology, 2023, 19(3): 157–163. DOI: 10.11731/j.issn.1673-193x.2023.03.023. [9] MA Q J, ZHANG Q, CHEN J C, et al. Effects of hydrogen on combustion characteristics of methane in air [J]. International Journal of Hydrogen Energy, 2014, 39(21): 11291–11298. DOI: 10.1016/j.ijhydene.2014.05.030. [10] HUANG Y, CHEN J, ZHANG Q, et al. Effects of hydrogen addition on the confined and vented explosion behavior of methane in air [J]. Journal of Loss Prevention in the Process Industries, 2014, 27(1): 65–73. DOI: 10.1016/j.jlp.2013.11.007. [11] BOURAS F, ATTIA M E H, KHALDI F, et al. Control of methane flame properties by hydrogen fuel addition: application to power plant combustion chamber [J]. International Journal of Hydrogen Energy, 2017, 42(13): 8932–8939. DOI: 10.1016/j.ijhydene.2016.11.146. [12] 刘振翼, 李嘉璐, 李鹏亮, 等. 高温高压下N2和CO2对CH4/C2H6/C3H8混合气抑爆效果研究 [J]. 北京理工大学学报, 2023, 43(2): 111–117. DOI: 10.15918/j.tbit1001-0645.2022.048.LIU Z Y , LI J L, LI P L, et al. Study on the explosion suppression effect of N2 and CO2 on CH4/C2H6/C3H8 mixtures at high temperature and high pressure [J]. Transactions of Beijing Institute of Technology, 2023, 43(2): 111–117. DOI: 10.15918/j.tbit1001-0645.2022.048. [13] LUO Z, WEI C, WANG T, et al. Effects of N2 and CO2 dilution on the explosion behavior of liquefied petroleum gas (LPG)-air mixtures [J]. Journal of Hazardous Materials, 2021, 403: 123843. DOI: 10.1016/j.jhazmat.2020.123843. [14] CHEN D, YAO Y, DENG Y. The influence of N2/CO2 blends on the explosion characteristics of stoichiometric methane-air mixture [J]. Process Safety Progress, 2018. DOI: 10.1002/prs.12015. [15] MITU M, PRODAN M, GIURCAN V, et al. Influence of inert gas addition on propagation indices of methane-air deflagrations [J]. Process Safety and Environmental Protection, 2016, 102: 513–522. DOI: 10.1016/j.psep.2016.05.007. [16] LU C, LIU Y, WANG H B, et al. Experimental study of the effects of CO2/H2 on the characteristic features of methane/air bursts. [J]. Journal of Safety and Environment, 2018, 18(5): 1788–1795. DOI: 10.13637/j.issn.1009-6094.2018.05.024. [17] HU E, JIANG X, HUANG Z, et al. Numerical study on the effects of diluents on the laminar burning velocity of methane-air mixtures [J]. Energy and fuels., 2012, 26: 4242–4252. DOI: 10.1021/ef300535s. [18] SIOU Y W, NUNG K L, CHI M S. Effects of flammability characteristics of methane with three inert gases [J]. Process Safety Progress, 2010, 29(4): 349–352. DOI: 10.1002/prs.10411. [19] LIANG Y, ZENG W, HU E. Experimental study of the effect of nitrogen addition on gas explosion [J]. Journal of Loss Prevention in the Process Industries, 2013, 26(1): 1–9. DOI: 10.1016/j.jlp.2012.08.002. [20] LI M H, XU J C, WANG C J, et al. Thermal and kinetics mechanism of explosion mitigation of methane-air mixture by N2/CO2 in a closed compartment [J]. Fuel, 2019, 255. DOI: 10.1016/j.fuel.2019.115747. [21] BENEDETTO A D, SARLI V D, SALZANO E, et al. Explosion behavior of CH4/O2/N2/CO2 and H2/O2/N2/CO2 mixtures [J]. International Journal of Hydrogen Energy, 2009. DOI: 10.1016/j.ijhydene.2009.05.120. [22] WEI H, XU Z, ZHOU L, et al. Effect of hydrogen-air mixture diluted with argon/nitrogen/carbon dioxide on combustion processes in confined space [J]. International Journal of Hydrogen Energy, 2018, 43(31): 14798–14805. DOI: 10.1016/j.ijhydene.2018.06.038. [23] LI Y C, BI M S, HUANG L, et al. Hydrogen cloud explosion evaluation under inert gas atmosphere [J]. Fuel Processing Technology, 2018, 180: 96–104. DOI: 10.1016/j.fuproc.2018.08.015. [24] LI Y C, BI M S, YAN C C, et al. Inerting effect of carbon dioxide on confined hydrogen explosion [J]. International Journal of Hydrogen Energy, 2019, 44(40): 22620–22631. DOI: 10.1016/j.ijhydene.2019.04.181. [25] YAN C C, BI M S, LI Y C, et al. Effects of nitrogen and carbon dioxide on hydrogen explosion behaviors near suppression limit [J]. Journal of Loss Prevention in the Process Industries, 2020, 67: 104228. DOI: 10.1016/j.jlp.2020.104228. [26] BASCO A, CAMMAROTA F, SARLI V D, et al. Theoretical analysis of anomalous explosion behavior for H2/CO/O2/N2 and CH4/O2/N2/CO2 mixtures in the light of combustion-induced rapid phase transition [J]. International Journal of Hydrogen Energy, 2015, 40(25): 8239–8247. DOI: 10.1016/j.ijhydene.2015.04.092. [27] QIAO L, GU Y, DAHM W J A, et al. Near-limit laminar burning velocities of microgravity premixed hydrogen flames with chemically-passive fire suppressants [J]. Proceedings of the Combustion Institute, 2007, 31(2): 2701–2709. DOI: 10.1016/j.proci.2006.07.012. [28] QIAO L, KIM C H, FAETH G M. Suppression effects of diluents on laminar premixed hydrogen/oxygen/nitrogen flames [J]. Combustion and Flame, 2005, 143(1/2): 79–96. DOI: 10.1016/j.combustflame.2005.05.004. [29] HU E J, HUANG Z H, HE J J, et al. Measurement of laminar burning velocities and analysis of flame stabilities for hydrogen-air-diluent premixed mixtures [J]. Chinese Science Bulletin, 2009, 54(5): 846–857. DOI: 10.1007/s11434-008-0584-y. [30] QIAO L, GU Y, DAHM W, et al. A study of the effects of diluents on near-limit H2-air flames in microgravity at normal and reduced pressures [J]. Combustion and Flame, 2007, 151(1/2): 196–208. DOI: 10.1016/j.combustflame.2007.06.013. [31] AZATYAN V V, SHEBEKO Y N, SHEBEKO A Y.A numerical modelling of an influence of CH4, N2, CO2 and steam on a laminar burning velocity of hydrogen in air [J]. Journal of Loss Prevention in the Process Industries, 2010, 23(2): 331–336. DOI: 10.1016/j.jlp.2009.12.002. [32] 程方明, 葛汉漳, 邓军, 等. 金属丝网位置对氢气/甲烷/空气火焰传播特性的影响 [J]. 安全与环境学报, 2024, 24(7): 2593–2600. DOI: 10.13637/j.issn.1009-6094.2023.1586.CHENG F M, GE H Z, DENG J, et al. Effect of wire mesh position on the propagation characteristics of hydrogen/methane/air premixed flames in pipelines [J]. Journal of Safety and Environment, 2024, 24(7): 2593–2600. DOI: 10.13637/j.issn.1009-6094.2023.1586. [33] 程方明, 苟籽妍, 罗振敏, 等. 掺氢比例对金属丝网阻抑掺氢甲烷燃烧火焰传播的影响 [J]. 爆炸与冲击, 2024, 44(4): 045402. DOI: 10.11883/bzycj-2023-0295.CHENG F M, GOU Z Y, LUO Z M, et al. Effect of hydrogen ratio on inhibition property of wire mesh to propagation of the flame by methane premixed with hydrogen. [J]. Explosion and Shock Waves, 2024, 44(4): 045402. DOI: 10.11883/bzycj-2023-0295. [34] 唐毅, 员亚龙, 李开源, 等. 球形非金属材料对甲烷掺氢爆炸抑制机理研究 [J]. 高压物理学报, 2022, 36(6): 182–189. DOI: 10.11858/gywlxb.20220609.TANG Y, YUAN Y L, LI K Y, et al. Explosion suppression performance of spherical non-metallic materials for methane hydrogen-doped syngas explosion. [J]. Chinese Journal of High Pressure Physics, 2022, 36(6): 182–189. DOI: 10.11858/gywlxb.20220609. [35] 陈晓坤, 马赛燕, 程方明, 等. 半封闭管道内CO2对掺氢甲烷燃爆特性的影响 [J]. 安全与环境学报, 2023, 23(12): 4279–4286. DOI: 10.13637/j.issn.1009-6094.2022.2087.CHEN X K, MA S Y, CHENG F M, et al. Influence of CO2 on the combustion and explosion characteristics of hydrogen-doped methane in a semi-closed pipe. [J]. Journal of Safety and Environment, 2023, 23(12): 4279–4286. DOI: 10.13637/j.issn.1009-6094.2022.2087. [36] SMITH G P, GOLDEN D M, FRENKLACH M, et al. An optimized detailed chemical reaction mechanism for methane combustion: GRI-95/0058 [R]. GRI Tech, 1995. [37] LIAO S Y, JIANG D M, CHENG Q. Determination of laminar burning velocities for natural gas [J]. Fuel, 2004, 83(9): 1247–1250. DOI: 10.1016/j.fuel.2003.12.001. [38] LAMOUREUX N, DJEBAILI-CHAUMEIX N, PAILLARD C E. Laminar flame velocity determination for H2-air-He-CO2 mixtures using the spherical bomb method [J]. Experimental Thermal and Fluid Science, 2003, 27(4): 385–393. DOI: 10.1016/S0894-1777(02)00243-1. [39] 胡二江, 何佳佳, 黄佐华, 等. 氢气-空气-稀释气混合气层流燃烧速度的测定和火焰稳定性分析 [J]. 科学通报, 2008(20): 2514–2525. DOI: 10.1360/csb2008-53-20-2514.HU E J, HE J J, HUANG Z H, et al. Determination of the laminar burning velocity and flame stability analysis of hydr ogen-air-diluted gas mixtures [J]. Chinese Science Bulletin, 2008(20): 2514–2525. DOI: 10.1360/csb2008-53-20-2514. [40] WU F, JOMAAS G, LAW C K. An experimental investigation on self-acceleration of cellular spherical flames [J]. Proceedings of the Combustion Institute, 2013, 34(1): 937–945. DOI: 10.1016/j.proci.2012.05.0600. [41] WANG Z H, WANG S X, WHIDDON R, et al. Effect of hydrogen addition on laminar burning velocity of CH4/DME mixtures by heat flux method and kinetic modeling [J]. Fuel, 2018, 232(15): 729–742. DOI: 10.1016/j.fuel.2018.05.146. [42] LIU G Y, ZHOU J H, WANG Z H, et al. Adiabatic laminar burning velocities of C3H8-O2-CO2 and C3H8-O2-N2 mixtures at ambient conditions. Part Ⅱ: mechanistic interpretation [J]. Fuel, 2020, 276: 117946. DOI: 10.1016/j.fuel.2020.117946. [43] SU B, LUO Z M, DENG J, et al. Comparative study on methane/air deflagration with hydrogen and ethane additions: Investigation from macro and micro perspectives [J]. Process Safety and Environmental Protection, 2023, 174: 561–573. DOI: 10.1016/j.psep.2023.04.030. -






 下载:
下载: