Effect of CO2 on the lower flammability limit of acetylene in O2/CO2 atmosphere
-
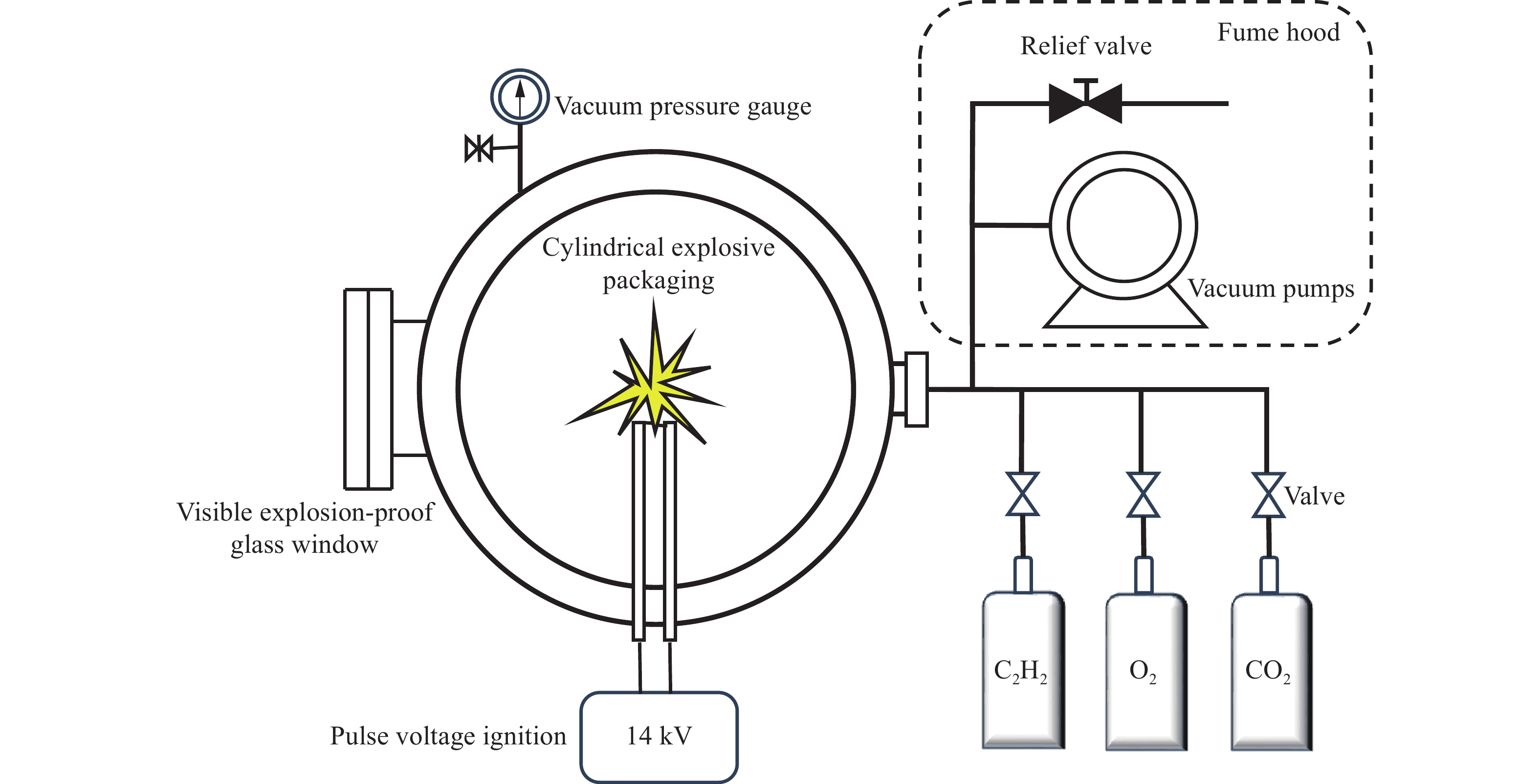
摘要: 为探究清洁燃料乙炔在O2/CO2气氛下的可燃下限,在5 L圆柱体爆炸反应装置中进行实验,测得了乙炔的可燃下限。随着CO2的体积分数从14%增加到85%,乙炔的可燃下限从2.64%增长到3.93%,在较小的范围内呈线性增加。烷烃、烯烃和炔烃的可燃下限依次降低,表明炔烃具有更大的燃烧范围。基于极限层流速度法计算模型,建立了适用于乙炔可燃下限的预测模型。通过实验数据验证了该模型的可靠性,采用该模型讨论了CO2的热力学、化学、输运效应对可燃下限的影响。结果表明:热力学效应的平均占比为64%,化学效应占比35%,输运效应占比1%。Abstract: Oxy-fuel combustion is one of the effective means to reduce greenhouse gases. To grasp the combustion characteristics of the clean fuel acetylene in O2/CO2 atmosphere and to investigate the effect of different CO2 volume fraction on the lower flammable limit of acetylene, the lower flammable limit of acetylene was experimentally measured in a 5 L cylindrical explosive reaction device. With the increase of CO2 volume fraction from 14% to 85%, the experimental value of the lower flammable limit of acetylene increased from 2.64% to 3.93%, which was linearly increased in a small range. Compared with hydrocarbon fuels such as ethylene, ethane, and propylene, the lower flammability limit of alkanes, olefins, alkynes decrease sequentially, indicating that alkynes have a larger combustion range and a higher hazard factor. Based on the calculation model of limiting laminar burning velocity method, a prediction model applicable to the lower flammability limit of acetylene was established. Through the verification of experimental data, the average absolute error of this prediction model using the USC Ⅱ combustion reaction mechanism is at 0.52%, and the model is accurate and reliable. To explain the reason for the existence of the lower flammability limit from the perspective of the competition between the temperature rise of the heat generation from fuel consumption and the temperature drop of the heat dissipation from the expansion of the fuel body, this study examines the thermodynamic, chemical, and transport effects of CO2 on the lower flammability limit. The combustion reaction mechanism of USC Ⅱ is modified to incorporate the virtual substances FCO2, TCO2, and MCO2, and comparing the flammability limits of the three virtual substances as well as those of the five atmospheres of N2 and CO2. The thermodynamic, chemical and transport effects of CO2 on the lower flammability limit were discussed. The results show that the average proportion of thermodynamic effect is 64%, chemical effect is 35% and transportation effect is 1%.
-
Key words:
- acetylene /
- O2/CO2 /
- lower flammability limit /
- limiting laminar velocity method
-
表 1 乙炔常用燃烧反应机理
Table 1. Common combustion reaction mechanisms for acetylene
序号 机理名称 年份 反应/物种数量 1 USC Mech Ⅱ 2007 784/112 2 GRI-Mech 3.0 1999 325/53 3 Davis 2017 469/71 4 San Diego 2014 247/50 5 Wang 1999 529/75 表 2 自由基峰值摩尔分数比例
Table 2. Ratio of peak molar fractions of free radicals
稀释气
体积分数/%自由基峰值摩尔分数比 H_CO2∶H_FCO2 O_CO2∶O_FCO2 OH_CO2∶OH_FCO2 H_CO2∶H_MCO2 O_CO2∶O_MCO2 OH_CO2∶OH_MCO2 14 0.928 1.001 1.030 0.881 0.983 1.042 52 0.769 0.959 1.070 0.681 0.881 1.120 85 0.790 0.805 1.038 0.760 0.702 1.251 -
[1] 李旭东, 谭青博, 赵浩辰, 等. 碳达峰背景下中国电力行业碳排放因素和脱钩效应 [J]. 中国电力, 2024, 57(5): 88–98. DOI: 10.11930/j.issn.1004-9649.202306019.LI X D, TAN Q B, ZHAO H C, et al. Carbon emission factors and decoupling effects of China’s power industry under the background of carbon peak [J]. Electric Power, 2024, 57(5): 88–98. DOI: 10.11930/j.issn.1004-9649.202306019. [2] ZHAO F M, ROGERS W J, MANNAN M S. Experimental measurement and numerical analysis of binary hydrocarbon mixture flammability limits [J]. Process Safety and Environmental Protection, 2009, 87(2): 94–104. DOI: 10.1016/j.psep.2008.06.003. [3] 尹林虎, 任小荣, 马利云, 等. 乙炔生产工艺应用与推广 [J]. 江西化工, 2018(1): 39–41. DOI: 10.14127/j.cnki.jiangxihuagong.2018.01.014.YIN L H, REN X R, MA L Y, et al. Application and popularization acetylene production [J]. Jiangxi Chemical Industry, 2018(1): 39–41. DOI: 10.14127/j.cnki.jiangxihuagong.2018.01.014. [4] NGUYEN V G, DAGER B, CHHILLAR A, et al. Desirability-based optimization of dual-fuel diesel engine using acetylene as an alternative fuel [J]. Case Studies in Thermal Engineering, 2024, 59: 104488. DOI: 10.1016/j.csite.2024.104488. [5] LAWRENCE K R, ANCHUPOGU P, REDDYGARI M R, et al. Optimization of biodiesel yield and performance investigations on diesel engine powered with hydrogen and acetylene gas injected with enriched Jojoba biodiesel blend [J]. International Journal of Hydrogen Energy, 2024, 50: 502–523. DOI: 10.1016/j.ijhydene.2023.09.166. [6] HU X Z, YU Q B, SUN N, et al. Effects of high concentrations of CO2 on the lower flammability limits of oxy-methane mixtures [J]. Energy & Fuels, 2016, 30(5): 4346–4352. DOI: 10.1021/acs.energyfuels.6b00492. [7] HU X Z, YU Q B, SUN Y S. Effects of carbon dioxide on the upper flammability limits of methane in O2/CO2 atmosphere [J]. Energy, 2020, 208: 118417. DOI: 10.1016/j.energy.2020.118417. [8] SONG D E, HU X Z. Effects of CO2 on the flammability limits of ethane in O2/CO2 atmosphere [J]. Fuel, 2022, 324: 124543. DOI: 10.1016/j.fuel.2022.124543. [9] HU X Z, XIE Q H, YU Q B, et al. Effect of carbon dioxide on the lower flammability limit of propane in O2/CO2 atmosphere [J]. Energy & Fuels, 2020, 34(4): 4993–4998. DOI: 10.1021/acs.energyfuels.0c00601. [10] CHENG F M, LI B B, LUO Z M, et al. Effect of CO2 on the explosion limit parameters and kinetic characteristics of ammonia-hydrogen-air mixtures [J]. Journal of Loss Prevention in the Process Industries, 2024, 92: 105480. DOI: 10.1016/j.jlp.2024.105480. [11] TIAN Y, BAI M Q, LI Y X, et al. Effects of N2 and CO2 on the flammability of 2, 3, 3, 3-tetrafluoropropene at elevated temperatures [J]. Journal of Loss Prevention in the Process Industries, 2023, 83: 105024. DOI: 10.1016/j.jlp.2023.105024. [12] KIM T, BUKAR M, BASNET S, et al. Effects of O2 concentration of O2/CO2 co-flow on the flame stability of non-premixed coaxial jet flame [J]. Fuel, 2024, 371: 132114. DOI: 10.1016/j.fuel.2024.132114. [13] 陈肯, 张一泽, 孙肇林, 等. O2/CO2气氛下CH4/H2可燃下极限的实验研究 [J]. 冶金能源, 2019, 38(6): 31–36. DOI: 10.3969/j.issn.1001-1617.2019.06.008.CHEN K, ZHANG Y Z, SUN Z L, et al. Experimental study on the lower flammability limits of CH4/H2 in O2/CO2 atmosphere [J]. Metallurgical Energy, 2019, 38(6): 31–36. DOI: 10.3969/j.issn.1001-1617.2019.06.008. [14] KUMUK O. CO2, Ar, and He dilution effects on combustion dynamics and characteristics in a laboratory-scale combustor [J]. Fuel, 2024, 369: 131745. DOI: 10.1016/j.fuel.2024.131745. [15] BAZALAN B B. Effect pressure on the flammability limits of acetylene [D]. Pahang: Universiti Malaysia Pahang, 2012. [16] WANG H, YOU X Q, JOSHI A V, et al. High-temperature combustion reaction model of H2/CO/C1-C4 compounds [EB/OL]. [2024-09-23]. http://ignis.usc.edu/USC_Mech_II.htm. [17] XU K W, HE C L, YIN J Z, et al. Relevance of soot formation characteristics to equivalence ratio and CO2 addition of acetylene flame [J]. Powder Technology, 2022, 412: 117978. DOI: 10.1016/j.powtec.2022.117978. [18] SIST EN 1839: Determination of explosion limits of gases and vapours and determination of the limiting oxygen concentration (LOC) for flammable gases and vapours [S]. SIST, 2017. [19] ISO. ISO 6141: 2015 Gas analysis-contents of certificates for calibration gas mixtures [S]. Geneva: ISO, 2015. [20] PIO G, SALZANO E. Evaluation of safety parameters of light alkenes by means of detailed kinetic models [J]. Process Safety and Environmental Protection, 2018, 119: 131–137. DOI: 10.1016/j.psep.2018.07.024. [21] 梁容真. 单组分气体燃料在O2/CO2气氛下的可燃极限研究 [D]. 沈阳: 东北大学, 2020. DOI: 10.27007/d.cnki.gdbeu.2020.002827.LIANG R Z. Study on the flammability limit of single-component gas fuel at O2/CO2 atmosphere [D]. Shenyang: Northeastern University, 2020. DOI: 10.27007/d.cnki.gdbeu.2020.002827. [22] ZHANG Y, SHEN W F, ZHANG H, et al. Effects of inert dilution on the propagation and extinction of lean premixed syngas/air flames [J]. Fuel, 2015, 157: 115–121. DOI: 10.1016/j.fuel.2015.05.007. [23] HU E J, JIANG X, HUANG Z H, et al. Numerical study on the effects of diluents on the laminar burning velocity of methane-air mixtures [J]. Energy & Fuels, 2012, 26(7): 4242–4252. DOI: 10.1021/ef300535s. -







 下载:
下载: